Understanding pH Meter Basics and Applications
Explore the fundamentals of pH meters, from their definition and importance in measuring acidity or alkalinity to the components and working principles involved. Learn about pH measurement techniques, electrode technology, challenges faced, and the significance of accurate pH readings in various industries.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
SeminarPpt.com Seminar on pH Meter Submitted to: Seminarppt.com Submitted By Seminarppt.com
Table Contents Definition Introduction pH Measurement pH Electrodes Temperature Compensation Buffer Solutions Challenges of pH Meter Conclusion 2
Definition pH meter is an instrument used to measure acidity or alkalinity of a solution - also know as pH. 3
Introduction The quantitative information provided by the pH value expresses the degree of the activity of an acid or base in terms of hydrogen ion activity. The pH value of a substance is directly related to the ratio of the hydrogen ion [H+] and the hydroxyl ion [OH-] concentrations. 4
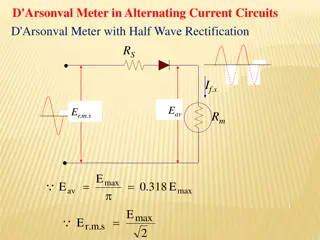
pH Measurement A rough indication of pH can be obtained using pH papers or indicators, which change color as the pH level varies. These indicators have limitations on their accuracy, and can be difficult to interpret correctly in colored or murky samples. More accurate pH measurements are obtained with a pH meter. 6
pH Measurement A pH measurement system consists of three parts: a pH measuring electrode, a reference electrode, and a high input impedance meter. The pH electrode can be thought of as a battery, with a voltage that varies with the pH of the measured solution. The reference electrode output does not vary with the activity of the hydrogen ion. 7
pH Measurement The pH electrode has very high internal resistance, making the voltage change with pH difficult to measure. The input impedance of the pH meter and leakage resistances are therefore important factors. In some cases, voltages can also be read for special applications or for use with ion- selective or Oxidation-Reduction Potential (ORP) electrodes. 8
pH Electrodes pH electrode technology has not changed much in the past 50 to 60 years. With all the technological advancements of the last 30 to 40 years, pH electrode manufacturing remains an art. The special glass body of the electrode is blown to its configuration by glass blowers. 9
pH Electrodes Not a terribly advanced nor high tech process but a very critical and important step in the electrode manufacturing. In fact, the thickness of the glass determines its resistance and affects its output. 10
Temperature Compensation Temperature compensation is contained within the instrument, because pH electrodes and measurements are temperature sensitive. The temperature compensation may be either manual or automatic. 11
Temperature Compensation With manual compensation, a separate temperature measurement is required, and the pH meter manual compensation control can be set with the approximate temperature value. With automatic temperature compensation (ATC), the signal from a separate temperature probe is fed into the pH meter, so that it can accurately determine pH value of the sample at that temperature. 12
Buffer Solutions Buffers are solutions that have constant pH values and the ability to resist changes in that pH level. They are used to calibrate the pH measurement system (electrode and meter). There can be small differences between the output of one electrode and another, as well as changes in the output of electrodes over time. Therefore, the system must be periodically calibrated. 13
Buffer Solutions Buffers are available with a wide range of pH values, and they come in both premixed liquid form or as convenient dry powder capsules. Most pH meters require calibration at several specific pH values. One calibration is usually performed near the isopotential point (the signal produced by an electrode at pH 7 is 0 mV at 25 C), and a second is typically performed at either pH 4 or pH 10. 14
Challenges of pH Meter No pH complications are created equal. The list below illustrates the types of problems that you can expect when measuring pH and how to handle them. Instrumentation is frequently the source of disturbance for pH systems, through repeatability error, measurement noise, or valve hysteresis. 15
Challenges of pH Meter In-line pH loops will oscillate, regardless of controller modes and tuning, if setpoints are on the steep parts of the titration curves. pH electrode submersion assemblies with unencapsulated terminations below the liquid surface will eventually have wet terminations. 16
Challenges of pH Meter Reagent control valves that are not close- coupled to the injection point on in-line systems will cause reagent delivery delays large enough to describe the tools of your trade n words that may seem foreign. You need either a flow meter or a seer to diagnose reagent delivery problems. 17
Challenges of pH Meter Flow feedforward signals should be multiplied by pH controller outputs and employed to operate reagent valves directly or to establish reagent flow control setpoints. Transportation delays to pH electrodes in analyzer houses will exceed mixing deadlines - such that increasing comfort in checking the electrodes is offset by decreasing comfort in checking trend recordings. 18
Challenges of pH Meter Injection electrodes should be preferred to sample holder assemblies whenever possible to reduce maintenance problems and improve response times - but not all injection electrodes are created equal. Large tanks are fine if you don't have to control them; use the volume upstream to reduce reagent consumption or downstream to reduce control error. If you can't make-up your mind where to use one, put it downstream. 19
Conclusion pH meter is an instrument used to measure acidity or alkalinity of a solution - also know as pH. pH is the unit of measure that describes the degree of acidity or alkalinity. It is measured on a scale of 0 to 14. A pH meter is an instrument used to measure hydrogen ion activity in solutions - in other words, this instrument measures acidity/alkalinity of a solution. 20
References Wikipedia.org Google.com Seminarppt.com Studymafia.org