Understanding Poisson-Fermi Theory of Ionic Solutions
Explore the Poisson-Fermi theory of ionic solutions, as discovered by Jinn-Liang Liu and Bob Eisenberg, which utilizes Fermi distribution and Yukawa potential to describe the interactions of ions in liquids. Learn about the challenges posed by Lennard-Jones potentials and the significance of local and global potentials in dealing with correlations in crowded conditions. Discover the role of Yukawa screened Coulomb potential in describing screening effects in ionic solutions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Poisson Fermi Theory of Ionic Solutions Bob Eisenberg March 17, 2018 Illinois Institute of Technology: Applied Math Rush University: Physiology and Biophysics Chicago Jinn-Liang Liu discovered role of SATURATION SATURATION using Fermi Distribution Fermi Distribution andYukawa Potential Yukawa Potential in Theories of Ionic Solutions Theories of Ionic Solutions 1
Poisson Fermi Formulation We couple the Screened Coulomb (Yukawa) Potential* and Far Field Poisson Electrostatics *to avoid the Lennard Jones Combining Rules that are Badly defined in Experiments and Simulations of Liquids 2 Poisson Fermi Formulation
Lennard Jones potentials are troublesome because they have combining rules that are ill defined in experiments, whether Lorentz-Berthelot or Kong. Combining parameters are likely to depend on ionic species, concentration and perhaps other variables. It is dangerous to have a model that depends sensitively on parameters that are unknown experimentally. 3 Poisson Fermi Formulation
Yukawa Screened Coulomb Potential We use Yukawa potential ? ? ? to describe Bjerrum/Debye Screening ? ? ? ? ? =??? ? (1) 4? ? ? Yukawa potential ? ? ? satisfies the differential equation ?2? ? ? + ??? ? ? = = ? ? ? (2) ? is the lowest order amplitude term in a general expansion of interaction of a pair of fermions The effective dielectric function of ?( ) is ??? ? is the Fermi-Thomas screening wave vector. ? 4 Poisson Fermi Formulation
Definition LOCAL POTENTIAL ? ?is defined by Poisson equation as ? -???0?2 ? ? = ??? ????(?) (4) ? Local Potential and Yukawa interaction must be joined to create a long range global potential to deal with correlations in high field or crowded conditions in which the size and valence of ions and the polarization of water play significant roles. Ions are often crowded where they are important 5 Poisson Fermi Formulation
Local Potential and Yukawa interaction are joined to create a long range Global Potential Global Potential is needed to deal with Correlations in high field or crowded conditions in which the size and valence of ions and the polarization of water are important. Ions are often crowded where they are important: DNA, ion channels, enzymes, binding proteins, electrodes, batteries, supercapacitors 6 Poisson Fermi Formulation
We introduce a GLOBAL POTENTIAL ? ? that is a convolution of Yukawa screened Coulomb potential and the Poisson local potential ? ? ? = ? ??? ? ? ? ? d? (5) Multiply the Yukawa potential ? ? ? in its defining differential equation (2) by the local Poisson potential ? ? and integrate to smooth the product, reducing the detail (and resolution) of the result. The smoothed global potential ? ? allows easier computation in a differential equation we will now use. 7 Poisson Fermi Formulation
GLOBAL POTENTIAL ? ? is a convolution of the Yukawa screened Coulomb potential and the local Poisson potential The global potential is a convolution eq. (5) and also a solution of the differential equation ?2?2? ? +? ? = ? ? (6) ? becomes approximate when we impose a finite domain for computation See Xie and Volkmer (2015) Comm Computational Physics 13:174-194. also Hildebrandt et al (2004) Phys. Rev. Lett. 93, 108104; Xie, Liu, and Eisenberg, Phys. Rev. E (2016) 94, 012114; using numerical methods in Xie, et al, (2012) 34:B107-B126. 8 Poisson Fermi Formulation
GLOBAL POTENTIAL ? ? combines Yukawa Screened Coulomb and Poisson Far Field ? (4) -???0?2 ? ? = ??? ????(?) ? (6) ?2?2? ? +? ? = ? ? Eq. (4) and (6) give the fourth order equation ?2???0?4? ? +???0?2? ? = ??? (7) which is best solved as a pair of second order differential equations, we think Liu and Eisenberg (2015) Phys Rev E 92: 012711, alsohttps://arxiv.org/pdf/011506.005953 Liu and Eisenberg (2018) J Chem Phys 148:054501, also https://arxiv.org/abs/1801.03470 https://arxiv.org/pdf/011506.005953 https://arxiv.org/abs/1801.03470 9 Poisson Fermi Formulation
We move from the potential to Free energy using a functional formulation but not yet dissipation 10 Poisson Fermi Formulation
Free energy formulation with functionals F(C, ?) = ) = ??? (C, ?)+ )+???(C), (8) ? ? ??? ?? ?? ) = ? ??? (C, ?) = ? ??? ?? + (9) ? ????? ? ? +? ? ??? ? (10) ???(C) = ??T ?? ? ?? ????(?) ?? ?? ? Here ???(C) is our (saturating) entropy functional embodying the entropy of all species ??and their steric interactions; ???(C,?) is our electrostatic functional; the sum over index ?is ?+? ?+? ? Average Volume ??= ?? And ? 1 is the inverse of the fourth order self adjoint positive linear operator Fourth Order OperatorL=????????? ? + ??????? ? (11) ? (12) It is usually best numerically to rewrite the Loperator as a pair of second order operators. 11 Poisson Fermi Formulation
Taking the variation of? ?,? at ? recovers previous Crucial Multiscale Equation that links Atomic and Macroscales of Potential ????????? ? + + ??????? ? = =??? (13) (13) with ? ? = ? on the boundary. This equation is converted to two second order differential equations to make numerical treatments easier and more accurate. 12 Poisson Fermi Formulation
Poisson Fermi Formulation Saturation Phenomena Crucial in crowded systems, Near electrodes, DNA, in ion channels, binding proteins, Enzyme active sites, Batteries, Electrodes, Supercapacitors Ions are often crowded where they are most important Much less important in homogeneous bulk solutions 13 Poisson Fermi Formulation
Volume in our Hard Sphere Model ?,?,? ? ? + ? Spherical Water is Species Voids* are Species ? + ? Spherical Ions are Species ?+? (14) ????+ ??+? Volume ? ? Voids Ions + Water ?where ?? is the radius of the ??? species The volume of each sphere ??=? ??is the total number of spheres in the volume ?. ??? *Voids are needed to fill space and for actual computations, as we shall see, spheres cannot fill space. Leaving out spheres produces contradictions and severe Numerical difficulties 14 Poisson Fermi Formulation
Void Volume Fraction ? is a key measure of crowding* in the Fermi Distribution free energy per mole = the activity of voids ?+? ?????; ? ??+? = ? (15) ????? ? ? Ions + Water *Ions are usually crowded where they are important, Near DNA and Electrodes Near and in ion channels, binding proteins, enzyme active sites. 15 Poisson Fermi Formulation
Steric Potential ????? is Normalized* Activity of Voids Key parameter in the Fermi Distribution ????? ?? ????? ? ? (16) *Normalized with respect to the bulk homogeneous solution ?Bulk. Ions are usually crowded where they are important, near DNA and Electrodes near and in Ion Channels, Binding proteins, Enzyme Active Sites ?+? ?+? Bulk (17) ? ????Bulk ????? ? ? 16 Poisson Fermi Formulation
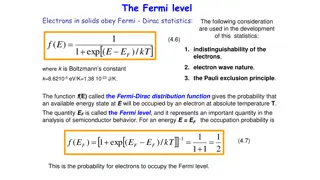
Steric Potential and the Fermi Distribution* ?????? ????????? = ????? ?? ?B= = reference potential of bulk ?? ? ? (18) Determines the Fermi Distribution of Concentrations* ???? ??? ? + +?? ?? ????? ??? = =?? (19) where??= ?? ??T. for Ions and ? = ?,?, ,?. also Water ? = ? + ?. . Water net charge = ??+1= 0. *Fermi distribution saturates! 17 Poisson Fermi Formulation
Saturation Phenomena can be derived from the Free Energy Functionals Taking the variation of ? ?,? at ??? , yields saturating, Fermi like distributions that we like to write in terms of the Steric Potential = ????? ?? ?B; ; ?B=reference potential of bulk ? ? (20) that determines the Fermi Distribution of Concentrations ???? ??? ? + + ?? ?? ????? ??? = =?? where??= ????T (21) for allIons and also for Water? = ? + 1. Water net charge = ??+1= 0 ? = 1,2, ,?. 18 Poisson Fermi Formulation
Fermi Distribution Saturation ???? ??? ? + + ?? ?? ????? ??? = =?? where??= ????T All concentration functions ??? < 1 ?? ??? cannot exceed the maximum value for any arbitrary (or even infinite) potential 1 ?? Voids Spheres cannot fill space Treatments with water as spheres cannot be computed unless voids are included Proof Follows 19 Poisson Fermi Formulation
Physiologistsgive the following Saturating Distribution the name Boltzmann ???? ? ? = ?+??? ????? ? ??? ? ? p.503 of Hodgkin and Huxley. 1952. Quantitative description ... J. Physiol. 117:500-544. Bezanilla, Villalba-Galea J. Gen. Physiol (2013) 142: 575 Gating charge Boltzmann Distribution* of Physicists ??? ????? does NOT Saturate ??? Fermi Distribution Saturates * Boltzmann. Berkeley Lectures on Gas Theory , 1904 (!) 20 Poisson Fermi Formulation
Proof: System Must Contain Voids Consider a system without voids, i.e., with ??+?= ? Let s try to fill the volume (1) with ions ?,?,? ? at concentrations ??? and (2) with the single water species K+1 and then use a Fermi Distribution. We will find a contradiction. We can use a Fermi Distribution only if we include voids. 21 Poisson Fermi Formulation
Proof: System Must Contain Voids Consider a system without voids, i.e., with ??+?= ? We will find a contradiction. Fermi Distribution for Spheres Requires Voids 22 Poisson Fermi Formulation
Consider a system without voids, i.e., with ??+?= ??+?= ? If the system is filled with spheres, with zero voids, then the volume fraction of voids is zero: ?????; ? = ? ?+? ????? ?????; ? = ? = ? (22) ? Ions + Water ?+? (23) ? = ????? ? 23 Poisson Fermi Formulation
Consider a system without voids, i.e., with ??+?= ??+?= ? If the system is filled with spheres, with zero voids, then the volume fraction of voids ?????; ? = ? ? ?+?????? = ? ?????? ????????? = ?????????? ??? ?? ????;? = ln ?B=ln 0 ?B ? ?????; ? Ions + Water ?????? ????????? = ?????????? ??? ?? ????;? = (24) 24 Poisson Fermi Formulation
Consider a system without voids, i.e., with ??+?= ??+?= ? If the system is filled with spheres, without voids, then the volume fraction of voids ????? = ?????; ? = ? is zero ? ?????? ????????? = ?????????? ???? ???????;? = (25) ????? ? ? + +?? ? ???? (26) ??????? ??? = =?? ??? = =? ? ??? =0 contradicts our original assumption of general ??? 25 Poisson Fermi Formulation
Proof Consider a system without voids, i.e., with ??+?= ??+?= ? Conclusion: We must have voids if we use a Fermi Distribution But we only need the Total Void Volume, or Volume Fraction No other details are needed about the voids 26 Poisson Fermi Formulation
Any Questions ? 27 Poisson Fermi Formulation