Understanding Proton NMR Spectroscopy Analysis
Explore the structural elucidation using NMR spectroscopy, from integral ratios to coupling constants, helping differentiate chemical environments based on proton signals in organic compounds. Gain insights into interpreting multiplets and coupling constants in NMR spectra.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
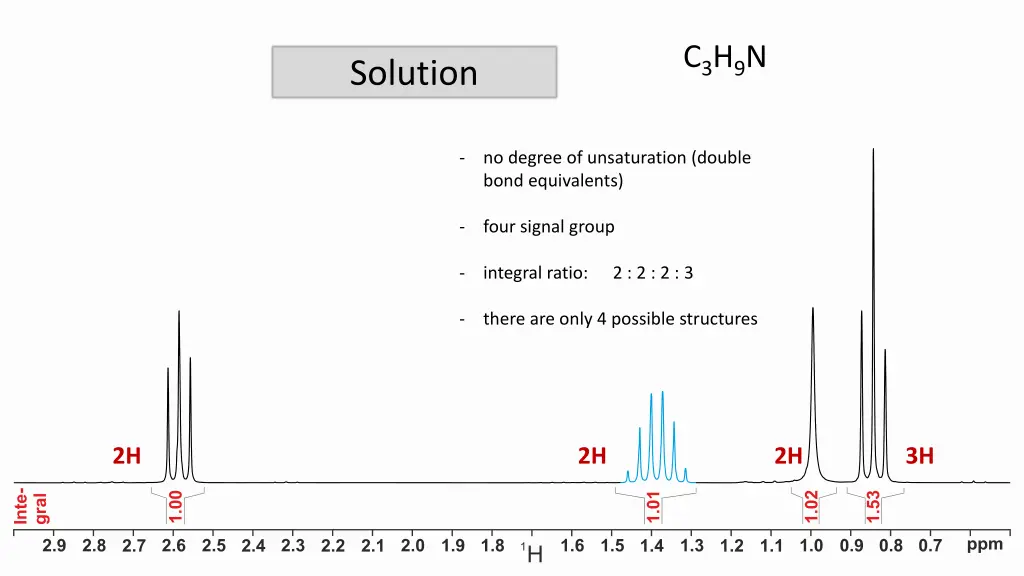
C3H9N Solution - no degree of unsaturation (double bond equivalents) - four signal group - integral ratio: 2 : 2 : 2 : 3 - there are only 4 possible structures 2H 2H 2H 3H
In which of the four possible isomers do four chemically distinguishable protons occur in the measured integral ratio? 1H distribution expected measured H3C N CH3 Trimethylamine 9H One signal group only. H3C H3C 6H 2H 1H CH NH2 Isopropylamine Only three signal groups. 3H (triplet) 2H (singlet) 2H (sextet ?) 2H (triplet) H3C H 3H 3H 2H 1H Four signal groups but not the measured integral ratio. N CH3 N-Ethylmethylamine H3C CH2 NH2 2H H2C 2H 2H 3H 3H 2H 2H 2H Four signal groups, integral ratio as measured. n-Propylamine CH2 H3C
The constitution could be deduced solely from the number of signal groups and the integral ratio between the signal groups. NH2 H2C CH2 The protons and the corresponding multiplets are labelled here with the same colours. But how did we made this assignment? H3C How do you explain the multiplet at about 1.4 ppm? NH2 2H H2C 2H 2H 3H CH2 H3C
Because of the integral of 3 the methyl group is easiest to assign. 0.84 ppm NH2 H2C The two protons of the neighbouring methylene group cause the splitting to form the triplet. CH2 H3C 2H 2H 2H 3H
The distance between two adjacent lines of the triplet is the coupling constant J1between the methyl and the methylene protons. 0.84 ppm NH2 H2C CH2 H3C J1 J1 2H 2H 2H 3H
The coupling constant J1should now naturally also appear in the multiplet of the methylene protons at approx. 1.4 ppm. NH2 J2 H2C CH2 At a distance of three bonds, there are two more equivalent methylene protons. The vicinal coupling constant J2should not differ much from J1. However, J1and J2are not identical. H3C J1 2H 2H 2H 3H
Theoretically, we expect a triplet of quartets with a total of 12 lines for the multiplet at 1.4 ppm. Six lines only are visible. NH2 J2 H2C CH2 When simulating the multiplet, it is a little clearer to start with the triplet splitting by the second methylene group. For J2, a coupling constant of 7.3 Hz is assumed in this simulation. H3C J1 2H 2H 2H 3H
The colours of the three lines have been slightly modified to distinguish them. Let's now shift them a little bit in the vertical direction to provide space for the splitting caused by the methyl protons. NH2 J2 H2C CH2 H3C J1 2H 2H 2H 3H
The three equivalent methyl protons now create a quartet from each line of the triplet. The coupling constant J1was assumed here to be 7.1 Hz, a little bit smaller than J2. NH2 J2 H2C CH2 H3C J1 2H 2H 2H 3H
There are actually 12 lines in total. Let's add lines with nearly identical chemical shifts. NH2 J2 H2C Because of the different values for J1and J2, the addition is not perfect. But the small differences cannot be resolved within the natural linewidth of the NMR signals and we get a pseudo-sextet in the integral ratio 1 : 5 : 10 : 10 : 5 : 1. CH2 H3C J1 2H 2H 2H 3H
J3 There are three bonds between the protons of the second methylene group and the protons of the amino group. We actually expect a vicinal coupling constant J3. NH2 J2 H2C However, the protons of this methylene group appear as a triplet, just like the protons of the methyl group. With the assumption of J3= 0 Hz, the triplet can be explained well. CH2 H3C J1 But why should we miss the vicinal coupling constant J3? 2H 2H 2H 3H
If OH and NH groups are involved, there often seems to exist no coupling pathway. The reason is the rapid chemical exchange of these protons. Let us assume two separate molecules of n-propylamine. We focus on the magnetic orientation of the protons of the amino group only. Statistically, there are almost as many protons with m = as with m = - . H CH3 N H2C H H2C CH2 H CH2 N H H3C H: m = H: m = -
Due to Brownian molecular motion, the amino groups of two molecules occasionally come into contact. CH3 H2C H CH2 H N H N H H2C H CH3 CH2 N H2C H3C H H2C CH2 H CH2 N H H3C H: m = H: m = -
After a small shift of the binding electrons, there are protons with other magnetic orientation bound to the nitrogen. The magnetic orientation of each individual proton remains unchanged. CH3 H2C H CH2 H N H N H H2C CH2 H3C H: m = H: m = -
After the molecules have separated again, the magnetic orientation of the protons of the amino group is different compared to the initial state. CH3 H2C H CH2 H N H N H H2C CH2 H3C H: m = H: m = -
After the molecules have separated again, the magnetic orientation of the protons of the amino group is different compared to the initial state. Repeating the chemical exchange fast enough results in permanent random change of m of the amino group protons. The effect is identical to broadband decoupling used to suppress carbon-proton-coupling when measuring carbon spectra. CH3 H2C The coupling between the amino group and the neighbouring methylene group becomes undetectable. CH2 N H H H H N H2C H: m = CH2 H3C H: m = -
A final remark The reality is more complex Have a look. The intensity ratio of this triplet is not perfectly 1 : 2 : 1. We must ignore the underlying effect here in the interest of a simple explanation of the multiplets. A detailed explanation can be found in the Problem of the month February 2021 . Generally, the very difficult to explain rule on the next page applies. Don t worry for the moment if the reasons behind it are not apparent.
A final remark The reality is more complex As soon as an asymmetrically substituted ethane is recognized as a structural fragment within an achiral compound, the methylene protons of this ethane fragment are always chemically equivalent and always magnetically non-equivalent. A H H A` X C C Y Why achiral? That s very simple. Within chiral compounds the methylene protons are chemically non- equivalent, which means, the question of magnetic equivalence doesn t appear. HH X X`
Contributions Spectrometer time TU Munich Measurements Rainer Hae ner Discussions and native English language support Compilation Alan Kenwright Dieter Str hl Rainer Hae ner More exercises
