Understanding Quantum Physics and Atomic Orbitals
Explore the fascinating world of quantum physics with a focus on atoms and their orbitals. Learn how electrons move between energy levels, the significance of ground states, and the emission and absorption of photons in these energy transitions. Delve into examples involving hydrogen energy levels and calculations of wavelength and frequency for emitted photons. Discover the energy required to ionize hydrogen atoms and more in this insightful exploration of quantum physics.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
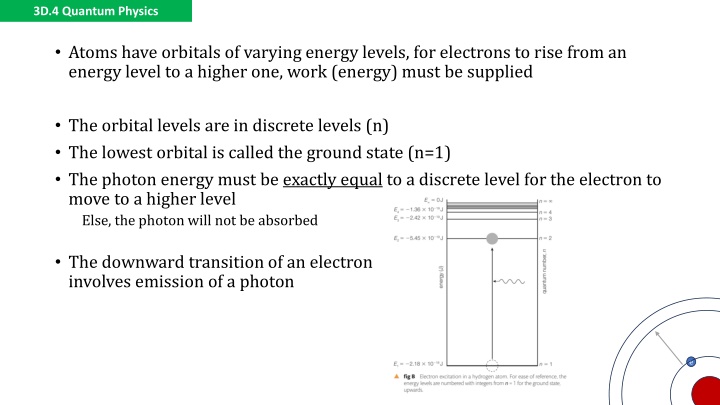
3D.4 Quantum Physics Atoms have orbitals of varying energy levels, for electrons to rise from an energy level to a higher one, work (energy) must be supplied The orbital levels are in discrete levels (n) The lowest orbital is called the ground state (n=1) The photon energy must be exactly equal to a discrete level for the electron to move to a higher level Else, the photon will not be absorbed The downward transition of an electron involves emission of a photon e
3D.4 Quantum Physics Appreciation: Hydrogen energy levels
3D.4 Quantum Physics Example:an electron in a hydrogen atom makes a transition from the n=2 energy level to the ground level (n=1). Find the wavelength and frequency of the emitted photon.
3D.4 Quantum Physics Example:an electron in a hydrogen atom makes a transition from the n=2 energy level to the ground level (n=1). Find the wavelength and frequency of the emitted photon. First, let s find the energy difference 13.606 3.401 = 10.205 eV E = hf (10.205 ?? 1.602 10 19 ?/??) 6.63 10 34 ? ? = 2.46 1015 ?? ? = 3 108 2.46 1015 = 122 nm ? =
3D.4 Quantum Physics Example:an electron in a hydrogen atom makes a transition from the n=2 energy level to the ground level (n=1). Find the wavelength and frequency of the emitted photon. First, let s find the energy difference 13.606 3.401 = 10.205 eV E = hf (10.205 ?? 1.602 10 19 ?/??) 6.63 10 34 ? ? = 2.46 1015 ?? ? = 3 108 2.46 1015 = 122 nm ? =
3D.4 Quantum Physics Example:an electron in a hydrogen atom makes a transition from the n=2 energy level to the ground level (n=1). Find the wavelength and frequency of the emitted photon. First, let s find the energy difference 13.606 3.401 = 10.205 eV E = hf (10.205 ?? 1.602 10 19 ?/??) 6.63 10 34 ? ? = 2.46 1015 ?? ? = 3 108 2.46 1015 = 122 nm 13.606 eV is the energy required to ionize the Hydrogen atom (the electron has left the H+) , how many Joules is that ? ? =
3D.4 Quantum Physics Line spectra: Light is made of multiple wavelengths, we can obtain spectral line by exciting a gas
3D.4 Quantum Physics Intensity of radiation: Any emitter of light has an intensity ???? ?2 =????? ? ???? ? ????????? ??