Understanding Redox Half Reactions and Oxidation Numbers
Learn how to assign oxidation numbers and analyze redox reactions in the context of the Haber Process. Understand the concepts of oxidation, reduction, and half-reactions, ensuring conservation of mass and charge.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Topic: Redox Half reactions Do Now: Quiz assign oxidation numbers
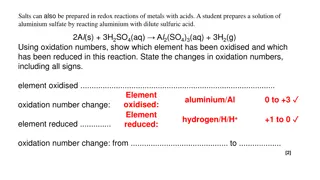
Now we know how to assign oxidation number we can look at redox rxns Haber Process N2(g)+ 3H2(g) 2NH3(g) Start by assigning oxidation numbers 0 0 -3 +1 N2(g)+ 3H2(g) 2NH3(g) What was oxidized? Reduced?
0 0 +1 -3 N2(g) + 3H2(g) 2NH3(g) Oxidation Is Losing electrons O I L 0 -1 -2 -3 -4 4 3 2 1 Began Ended N 0 -3 Reduction Is Gaining electrons R I G Gained 3 electrons = Reduced H 0 +1 Lost 1 electron = Oxidized
Why use the word reduced when electrons are gained? Electrons are Negative! Look how oxidation number changes Ex: Cl gains an electron Cl-1 oxidation # from 0 to -1; the # was reduced
Half Reactions Even though oxidation & reduction reactions occur together we write separate equations for each process and include # of e- gained/lost known as Half-Reactions
Half Reactions Half-reactions must demonstrate: conservation of mass & conservation of charge # atoms on left must = # atoms on right total charge on left must = total charge on right
0 0 -3 +1 N2(g) + 3H2(g) 2NH3(g) Oxidation = electrons lost (becomes more positive) So electrons are on the product side H lost 1 electrons, but since its NH3, there are 3 H so a total of 3 e- are lost H2 H+1 + 1e- 0 +1 1 and -1 equals 0 = OIL But something is wrong!
Remember H2 H+1 + 1e- Total Charge on left = Total Charge on right # atoms on left = # atoms on right H2 2H+1 + 1e- Something is still wrong! Charge is off now H2 2H+1 + 2e- 2 x +1 = +2 and -2 equals 0
0 0 -3 +1 N2(g) + 3H2(g) 2NH3(g) Reduction = electrons gained (becomes more negative) So electrons are on the reactant side Each N gained 3 electrons, but 2 N so N2 + 6e- N-3 0 + -6 2 X -3 = RIG 2
Oxidizing Agent: Substance being reduced Accepts electrons aids oxidation for another species Nitrogen is the Oxidizing Agent Reducing Agent: Substance being oxidized Loses electrons aids reduction for another species Hydrogen is the Reducing Agent
0 +2 -2 0 You Try: Mg + S MgS What is oxidized? What is reduced? Assign Oxidation Numbers Figure out change in oxidation numbers Mg: 0 to +2 = Oxidation S: 0 to -2 = Reduction
Now write the Half-Reactions 0 0 +2 -2 Mg + S MgS S is reduced: Mg is oxidized: Mg Mg+2 + 2e- S + 2e- S-2
0 0 +1 -1 +2 -1 Zn + 2HCl What is oxidized? Reduced? H2 + ZnCl2 Zn goes from 0 to +2 = oxidation H goes from +1 to 0 = reduction Cl goes from -1 to -1; No change
0 0 +1 -1 +2 -1 Zn + 2HCl H2 + ZnCl2 Write Half Reactions Zn 2H+1 + 2e- Zn+2 + 2e- H2
Taking it one step further
The steps 1. Assign oxidation numbers to all atoms in equation 2. Determine elements changed oxidation number 3. Identify element oxidized & element reduced 4. Write half-reactions (diatomics must stay as is, everyone you can write with their oxidation number only NH3 = N-3 but Cl2 stays Cl2 not Cl) Number electrons lost & gained must be equal; multiply half-reactions if necessary 5. 6. Add half-reactions; Transfer coefficients to skeleton equation 7. Balance rest of equation by counting atoms
+2 +5 -2 +1 +5-2 0 0 Cu + AgNO3 Cu(NO3)2 + Ag 1. Assign oxidation numbers to all atoms in equation 2. Determine elements changed oxidation number Cu 0 +2 Ag +1 0 N +5 +5 unchanged O -2 -2 unchanged 3. Identify element oxidized & element reduced Oxidation (OIL) =Cu Reduction (RIG) = Ag
+2 +5 -2 +1 +5-2 0 0 Cu + AgNO3 Cu(NO3)2 + Ag 4. Write half-reactions Cu Cu+2 + 2e- Ag+1 + 1e- 5. Number electrons lost & gained must be equal; multiply half- reactions if necessary 2(Ag+1 + 1e- Ag) 2Ag+1 + 2e- 2Ag Ag
+2 +5 -2 +1 +5-2 0 0 Cu + AgNO3 Cu(NO3)2 + Ag 6. Add half-reactions; Transfer coefficients to skeleton equation Cu Cu+2 + 2e- 2Ag+1 + 2e- +______________________ Cu + 2Ag+1 + 2e- 2Ag + Cu+2 + 2e- Cu + 2Ag+1 2Ag + Cu+2 2Ag Cu + AgNO3 2 original reaction: Cu(NO3)2 + Ag 7. Balance rest of equation by counting atoms 2