Understanding Sigmatropic Rearrangements and Cope Rearrangement in Organic Chemistry
Learn about sigmatropic rearrangements involving the shifting of a π-bond within a molecule and the interesting Cope rearrangement process. Dive into suprafacial and antarafacial processes, analysis based on FMO theory, and sigmatropic migration of carbon compounds. Explore the fascinating world of organic chemistry reactions and mechanisms.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
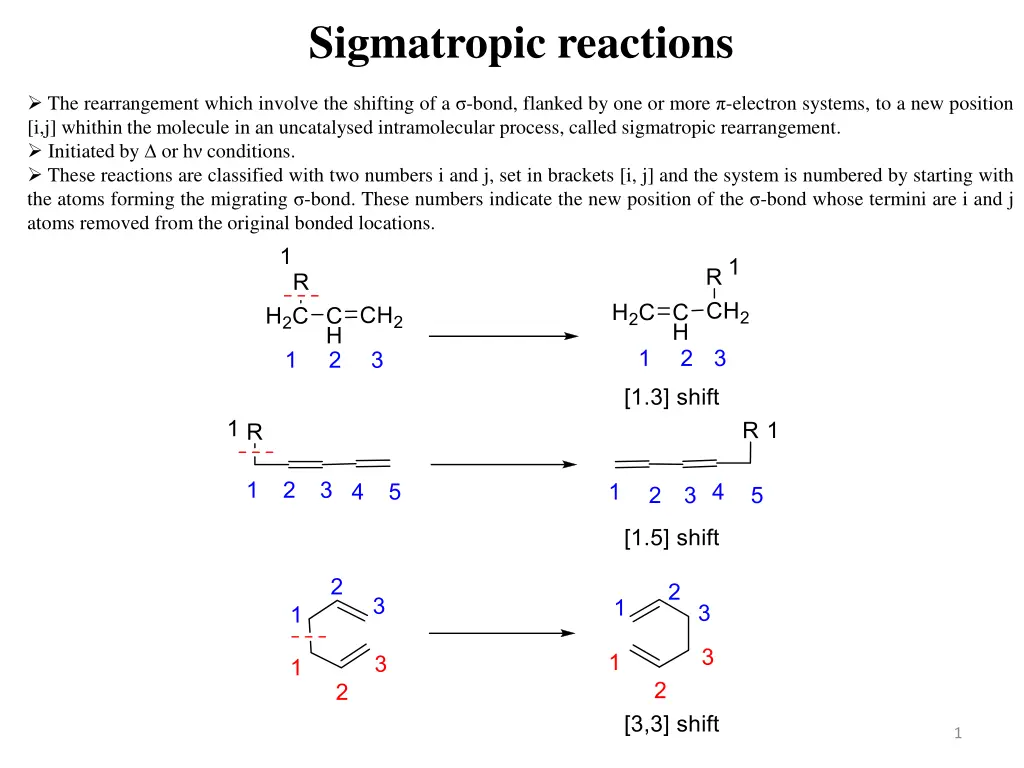
Sigmatropic reactions The rearrangement which involve the shifting of a -bond, flanked by one or more -electron systems, to a new position [i,j] whithin the molecule in an uncatalysed intramolecular process, called sigmatropic rearrangement. Initiated by or h conditions. These reactions are classified with two numbers i and j, set in brackets [i, j] and the system is numbered by starting with the atoms forming the migrating -bond. These numbers indicate the new position of the -bond whose termini are i and j atoms removed from the original bonded locations. 1
Suprafacial and Antarafacial processes When migrating -bond moves across the same face of the conjigated system, it is called a suprafacial process, whereas in the antarafacial process the migrating -bond is reformed on the opposite -electron face of the conjugated system. Due to steric reasons (geometrical fisibility) suprafacial migrations are more common than antarafacial shifts. However, with the lengthening of the conjugated system, sometimes antarafacial shifts are possible. 2
Analysis of sigmatropic reactions (FMO) To analyse sigmatropic rearrangement, it is to assume that the migrating bond undergoes homolytic cleavage resulting in the formation of a pair of radicals. As bonding characters are to be maintained throughout the course of the rearrangement, the bonding interaction will be in between the HOMO s of the two species produced by this cleavage. Each HOMO contains one unpaired electron. 3
Analysis of sigmatropic reactions (FMO) 1,5-sigmatropic reactions 4
Cope rearrangement Substrate: 1,5-diene It is an intramolecular process proceeding through a cyclic T. S. It is an example of a [3,3]-sigmatropic shift in which a chair-like T. S. is energetically preffered to a boat-like structure. 8
Cycloaddition reactions of Ketenes Ketenes undergo (2+2) thermal cycloaddtition reactions. Unlike other alkenes, ketenes undergo suprafacial-antarafacial process (2s+2a) of cycloaddition with alkene partner. This stereochemical mode can be possible only when two double bonds are perpendicular to each other. 9
Cycloaddition reactions of Ketenes cis-alkenes are more reactive than trans-alkenes in (2+2) cycloaddition with ketenes. Disubstituted ketenes react slowly due to steric hindrance. Bulkier substituent on ketene will be on more hindered face of cyclobutanone. Stereochemistry of alkenes remain maintained during the reaction. 10
Cheletropic reactions Two -bonds are formed or brokened from the same atom in concerted manner. For example the reaction of singlet carbene and SO2 with olefines. Examples and explaination for cheletropic reactions 11