Understanding the Concept of Equilibrium in Chemistry
Explore the concept of equilibrium in chemistry through a series of images and explanations covering topics such as dynamic equilibrium, phase equilibrium, solution equilibrium, reversible reactions, and more. Enhance your understanding of chemical equilibrium and its importance in various processes.
Uploaded on | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Topic: EQUILIBRIUM Do Now:
Equilibrium = Balance Not necessarily equal 1 man and 1 man equal but not balanced
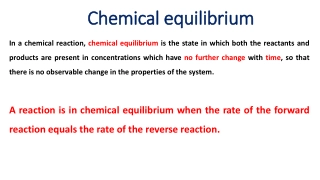
Chemical equilibrium Rate of forward reaction is EQUAL to the Rate of the reverse reaction Concentrations are not necessarily equal but they are constant, unchanging
N2(g) + 3H2(g) 2NH3(g) EQUILIBRIUM POINT Concentration H2 NH3 N2 Time
Dynamic Equilibrium macroscopic level looks like nothing is happening microscopic level lots going on
Phase Equilibrium phase changes are reversible processes H2O(l) H2O(g) H2O(l) H2O(s) same substance on both sides, only its phase is different NOTE: anytime a gas is involved, the container must be sealed for equilibrium to be reached- if not the gas will escape! Liquid Vapor Equilibrium
Solution Equilibrium saturated solution with some solid on the bottom of the container. As one molecule dissolves, one molecule precipitates (forms solid). Ex: Too much sugar in the coffee. - EX: CO2 in water CO2(g) CO2(aq) favored by high pressure & low temperature
Reversible Reactions Most chemical reactions are reversible. Reactants react to form Products, then the Products react to form Reactants. N2(g) + 3H2 (g) 2NH3(g) N2 reacts with H2 to form NH3 at the same time, NH3 is consumed and formsN2 and H2
Reversible Rxn vs Reaction that goes to completion NaOH + HCl --> NaCl + H2O
equilibrium can be changed or affected changes in concentration, pressure, temperature affect forward & reverse reactions differently composition of equilibrium mixture will shift to accommodate these changes
Le Chateliers Principle When a system at equilibrium is subjected to a stress, the equilibrium will shift in the direction which tends to relieve the stress. stress = change in concentration, volume, pressure, or temperature
Increase Concentration INCREASING the concentration causes the equilibrium to shift AWAY from the substance that was added.
N2 + 3H2 2NH3 At equilibrium Increase N2 In order to get back to equilibrium, what has to happen? Need more products! What happens to the amount of H2? Rxn shift to the RIGHT
N2 + 3H2 2NH3 Increase NH3 Need more N2 and H2 so reaction shifts to the left
DECREASING the concentration causes the equilibrium to shift TOWARD the substance that was removed.
N2 + 3H2 2NH3 Decrease NH3 Need more NH3 so reaction shifts to the right
Changes in Temp shift in the direction opposite of the heat term exothermic reaction: A + B C + D + heat If temperature, system shifts to consume heat so shifts to left so endothermic rxn favored endothermic reaction: A + B + heat C + D If temperature, system shifts to consume heat so shifts to right so exothermic rxn favored
!! PRESSURE !! N2(g) + 3H2(g) 2NH3(g) Increase PRESSURE, shift toward the side of the equation that has LESS MOLES of GAS. Decrease Pressure, shift toward the side of the equation that has MORE MOLES of GAS.
VOLUME Increasing Volume decreases pressure. Equilibrium will shift toward greater number of moles of gas. Decreasing Volume increases pressure. Equilibrium will shift toward smaller number of moles of gas.
Reactions that go to completion equilibrium can not exist Formation of a gas which escapes H2CO3 (aq) CO2(g) + H2O (l) Formation of water HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) Formation of a precipitate (insoluble compound) NaCl (aq) + AgNO3(aq) AgCl (s) + NaNO3 (aq)
A + B C + D Stress Equil. Shift [A] [B] [C] [D] DEC INC INC right [A] ______ INC INC DEC [D] ______ left [C] ______ DEC DEC INC right [B] ______ INC DEC DEC left