Understanding the Difference: Evaporation vs. Boiling in Diffusion Processes
Explore the disparities between evaporation and boiling processes, dive into the concept of diffusion, and grasp the movement of particles from high concentration areas to low concentration areas. Discover the factors influencing diffusion rates and enhance your knowledge in this fundamental scientific phenomenon.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
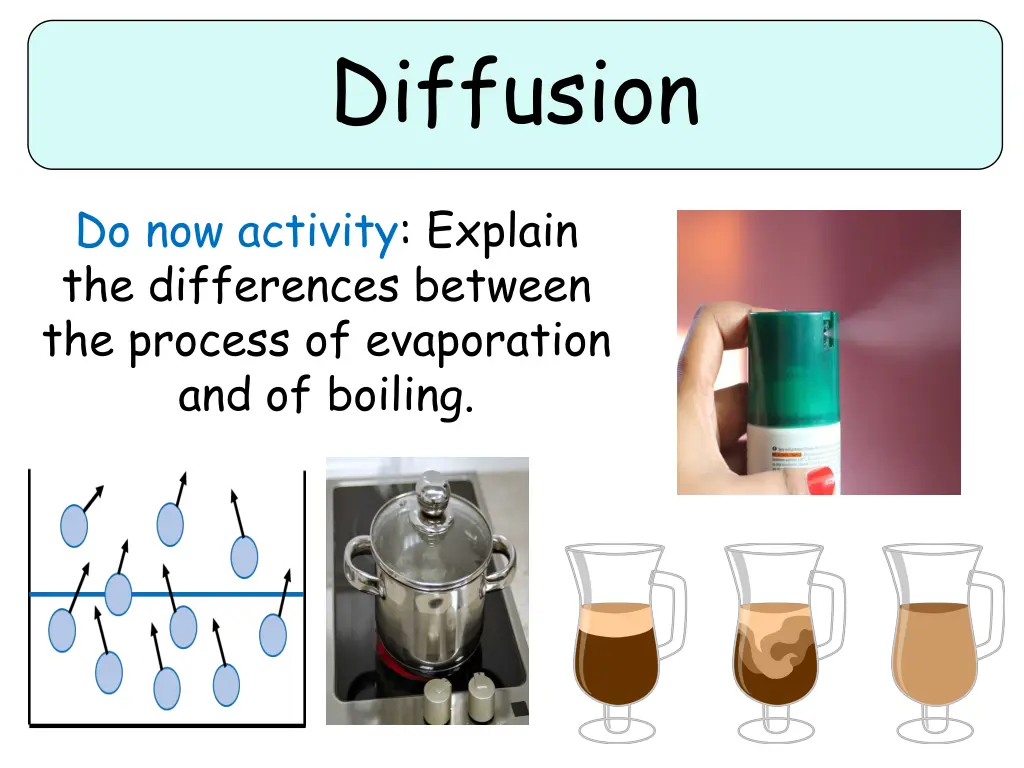
Diffusion Do now activity: Explain the differences between the process of evaporation and of boiling.
Progress indicators GOOD PROGRESS: Recall the definition of diffusion Describe evidence for diffusion OUTSTANDING PROGRESS: Use the particle model to explain diffusion
When you have just sprayed perfume, the concentration of perfume particles in that area is very high. Air particle Perfume particle Diffusion is the movementof the smelly particles, through particles of air, to an area where they are at a LOWER concentration.
Definition of diffusion: The spreading out of particles of a gas, or any substance in solution, resulting in the net movement of particles from an area of high concentration to an area of low concentration.
Diffusion Task: Copy and complete the following sentences: Diffusion is the _____________ of particles from any area where they are in a ____ __________ to an area where they are in a ______ ___________.
Self-assessment: Diffusion is the movement of particles from any area where they are in a high concentration to an area where they are in a low concentration.
4 3 1 2 Task: Draw the diagrams above and match the correct statements to the correct diagram to demonstrate the sequence of diffusion The particles are now nearly diffuse The particles are separate when first put together The particles are fully diffuse They slowly start to mix due to their random motion
The particles are separate when first put together They slowly start to mix due to their random motion The particles are now nearly diffuse The particles are fully diffuse
Think > Pair > Share! Size of particles State What factors might affect the rate of diffusion? Concentration of particles Temperature
Think > Pair > Share: How do you think temperature might affect the rate of diffusion? http://lab.concord.org/embeddable.html#interactives/sam/diffusion/2-temperature.json Key Words: thermal, collide, higher, diffusion, move In summary, the _________ the temperature the quicker the rate of _________. This is due to the fact the an increase temperature means the particles are supplied with more _______ energy. The more energy the particles have, the more they _______ around and will _______ more often. This means diffusion will happen much more rapidly.
Self-assessment: In summary, the _________ the temperature the quicker the rate of _________. diffusion higher This is due to the fact the an increase temperature means the particles are supplied with more _______ energy. The more energy the particles have, the more they _______ around and will _______ more often. collide thermal move This means diffusion will happen much more rapidly.
Particle Size Larger, heavier particles diffuse more slowly than small, light particles.
State Think > Pair > Share: Do you think diffusion happens more quickly in gases, liquids or solids? Explain your answer! Diffusion occurs more quickly in gases; this is because the particles are far apart from each other and so can travel long distances without hitting another particle. In the liquid state, particles are packed more closely together. Therefore, diffusion is slower in liquids.
Diffusion happens in liquids & gases too ... why not in solids? (clue: the particle model)
Liquid Solid Gas Task: Copy and complete: The particles in a solid do not move, whereas particles in a liquid and a gas do move. Therefore, only particles that can move can The particles in a _______ do not move, whereas particles in a ________ and a _______ do move. Therefore, only particles that can move can _________. diffuse. Liquid gas diffuse solids
Plenary What did you learn this lesson? 3 facts 3 key words 1 question to test your peers
