Understanding the Impact of Chlorofluorocarbons (CFCs) on the Environment
Explore the introduction, sources, chemical reactions, and environmental impacts of Chlorofluorocarbons (CFCs) commonly known as Freon. Learn how CFCs contribute to global warming, ozone depletion, and their alternatives to protect the environment.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
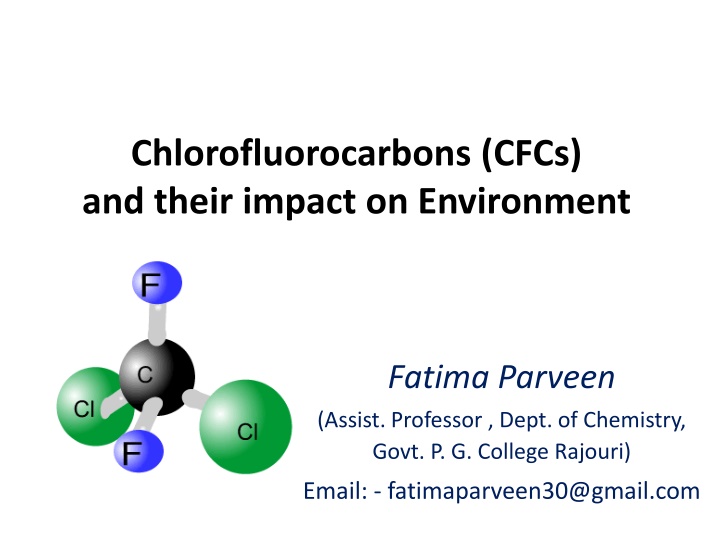
Chlorofluorocarbons (CFCs) and their impact on Environment Fatima Parveen (Assist. Professor , Dept. of Chemistry, Govt. P. G. College Rajouri) Email: - fatimaparveen30@gmail.com
Objectives CFCs Introduction Sources Chemical Reactions Environmental impacts CFCs alternatives
Introduction CFCs commonly known as Freon CFCs are Synthetic chemicals Contain fluorine, chlorine and carbon (no hydrogen) Have a similar structure to alkanes, with hydrogen replaced by fluorine and chlorine. E.g. Dichlorodifluoromethane (Freon 12)
Source CFCs are anthropogenic compounds that are released into the atmosphere, since 1930s because of their wide use in air-conditioning, refrigeration, insulations, propellants (in aerosol applications), and solvents and packing materials.
Environmental Impacts CFCs are greenhouse gases Absorb heat in the atmosphere, sending some of the absorbed heat back to the surface of the earth Contribute to global warming and climate change. UV radiation in stratosphere causes CFC molecules to dissociate into free radicals that contains one or more unpaired electrons.
A single chlorine atom can cause the conversion of thousands of ozone molecules to oxygen that leads to ozone depletion.
Ozone Depletion Source: Environmental Protection Agency, USA
Effects of Ozone Depletion Global Warming Increase in levels of skin cancer, cataracts, immune system impairment. Impact on crops and agriculture Impact on marine food cycle
CFC Alternatives Impact of Chlorofluorocarbon Replacements on Stratospheric Ozone Source: http://www.mp-docker.demon.co.uk/environmental_chemistry/topic_2b/page_4.html
CFCs, HCFCs and HFCs Ozone Depletion Potential of CFCs, HCFCs and HFCs Ozone Depletion Potential of CFCs, HCFCs and HFCs 1.2 ODP (Ozone Depletion Potential) 1 1 1 0.8 0.6 0.4 0.2 0.055 0.02 0 0 0 CFC-11 CFC-12 HCFC-22 Compound HCFC-123 HFC-32 HFC-128