Understanding Titration: Types, Applications, and Standardization
Explore the diverse world of titration, including different types like acid-base and precipitation, applications such as neutralization titration, and procedures for standardization using examples like NaOH and HCl solutions.
Uploaded on | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
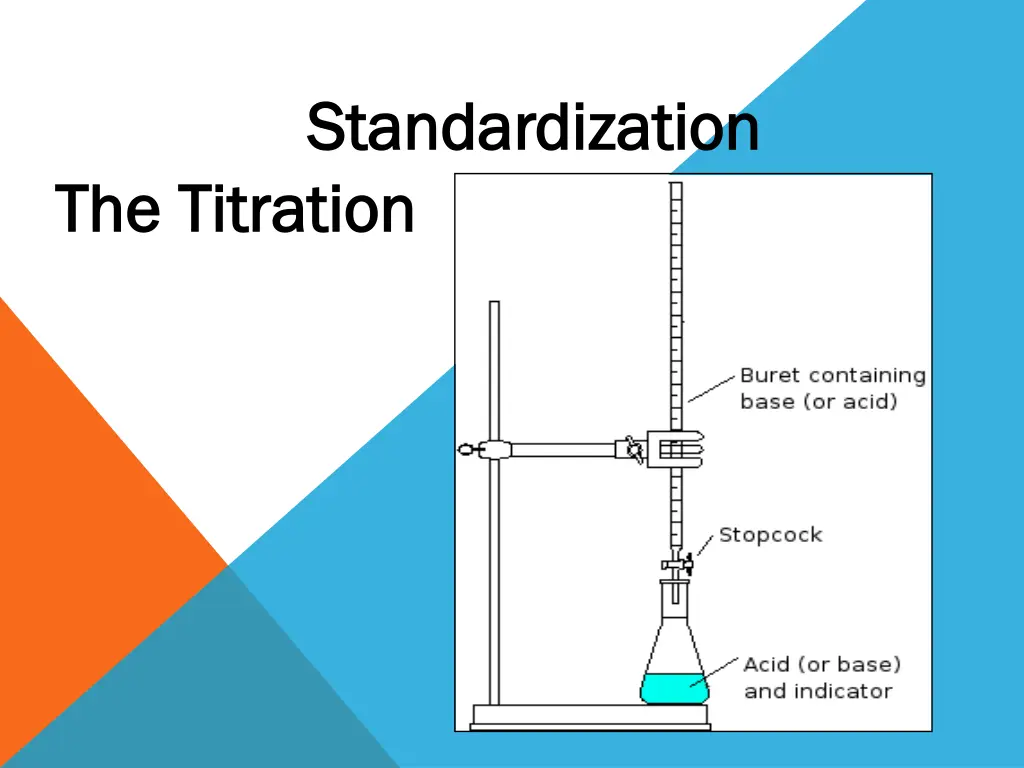
Standardization Standardization The Titration The Titration
TYPES OF TITRATION TYPES OF TITRATION 1-Acid base 2-Oxidation-reduction 3-Precipitation titration 4-Complex-formation titration
Applications of Neutralization Titration Applications of Neutralization Titration When a solution of a strong acid is mixed with solution of a strong base, a chemical reaction H+ (aq) + OH- (aq) H2O
Experiment Experiment 4 4 Preparation and standardization ( Preparation and standardization (0.1 ( (0.1 0.1 N) N) Na NaOH OH 1-Preparation of an (0.1N ) Na OH N = 0.1 N ) N ) HCL with HCL with ?? .???1000 ?? ???N = 0.1 V ml = 250 Eq. wt = 40 Wt = X = 1 Weigh (1gm ) of Na OH , Dissolve and dilute in volumetric flask with (250 ml ) by using distilled water .
2. Preparation 2. Preparation of (0.1N) N(HCl) = ??????????????? % of (0.1N) HCl HCl from concentrated from concentrated HCl ??? 1000 ?? .??????? HCl Specific gravity = 1.18 Eq.wt of HCl = 36.5 %38 ? . .. .. . N concentrated HCL = 12 To calculate the volume of concentrated HCL that should be taken to prepare 250 ml of (0.1N) HCL Conc.HCL Conc.HCL diluted HCL N1 V1 = N2 V2 12 X V1 = 0.1 X 250 V1 = 2.2 ml 2.2 ml should be taken from the concentrated HCL and should be diluted with distilled water in the volumetric flask with (250 ml ) to obtain ( 0.1 N) HCL ? of HCl is obtained from the reagent bottle diluted HCL
Standardization of ( Standardization of (0.1 0.1N ) N ) NaOH NaOH solution solution Procedure Procedure : : 1.Transfer (10.0 ml) of standard HCl solution to conical flask . 2. Add 1-2 drops of phenolphthalein(ph- ph)indicator it will be colorless . 3. Place a white sheet of paper under the flask to aid in the detection of any color change . 4. Fill the burette with the prepared NaOH solution . 5. Start titration by adding NaOH solution dropwise into the conical flask until the color of the solution is faint pink
6. The exact normality of NaOH is obtained from the following equation : HCl HCl + + NaOH NaOH NaCl NaCl + H + H 2 2O O N N1 1 V V1 1 (NaOH) ) = = N N2 2V V2 ( HCl 2






















