Understanding Titrations: Key Concepts and Equations
Explore the concept of titrations where a solution of known concentration is gradually added to another solution until a chemical reaction is complete. Learn about strong acid-strong base titrations and how to calculate molarity based on titration results.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Topic 1.5 Titrations
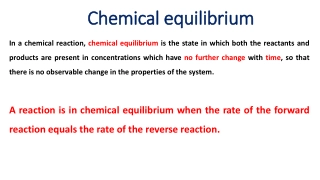
Titrations In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point the point at which the reaction is complete Indicator substance that changes color at the endpoint (hopefully close to the equivalence point) Slowly add base to unknown acid UNTIL The indicator changes color (pink) 4.7
Strong Acid-Strong Base Titrations 100% ionization! NaOH (aq) + HCl (aq) H2O (l) + NaCl (aq) OH- (aq) + H+(aq) H2O (l) No equilibrium 16.4
Titration equation The titration equation can be used for neutralization reactions (strong acid-strong base). NaOH (aq)+ HCl (aq) H2O(l) + NaCl (aq) Bb x Ca x Va = Ba x Cb x Vb Volume of base Balanced base coefficeint Concentration of base Concentratio n of acid Balanced acid coefficeint Volume of acid
A titration of a 25.00 mL sample of a hydrochloric acid solution of unknown molarity reaches the equivalence point when 38.28 mL of 0.4370 M NaOH solution has been added. What is the molarity of the HCl solution? NaOH(aq) + HCl(aq) NaCl(aq) +H2O(l) 0.6691 M HCl
A 50.00 mL sample of a sodium hydroxide solution is titrated with a 1.605 M solution of sulfuric acid. The titration requires 24.09 mL of the acid solution to reach the equivalence point. What is the molarity of the base solution? H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) + 2H2O(l) 1.547 M NaOH