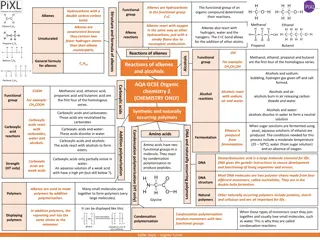
Unsaturated Hydrocarbons: Alkenes and Alkynes Overview
Learn about alkenes and alkynes, two types of unsaturated hydrocarbons that contain double or triple bonds. Explore their properties, naming conventions, isomers, and more in this informative guide. Understand the differences between alkenes and alkynes and how to distinguish isomers within these organic compounds.
Uploaded on | 3 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Topic: Alkenes & Alkynes (unsaturated hydrocarbons) Do Now: Name the following. Are the Isomers?
Unsaturated hydrocarbons: organic compounds containing one or more double or triple bonds can add more H by breaking the bond
Alkenes Another homologous series of hydrocarbons Each member contains at least one double covalent bond between C atoms So alkenes are unsaturated General formula = CnH2n
Properties of Alkenes Nonpolar low solubility in H2O fairly low melting and low boiling points More reactive than alkanes : double bond is site of reactivity
Naming Alkenes 1. Count longest carbon chain prefix tells # of C s in longest chain and add ene 1st member is C2H4: ethene H H C=C H H
3. If more than 4 Carbons give 1st carbon in double bond the lowest possible number when numbering the chain ] 1-butene 2-butene
If 2 double bonds = diene 1,3 pentadiene
Naming Branched-Chain Alkenes 1st carbon in Double bond gets lowest number Then name branches based on numbers assigned Branch name and number come first when naming Chain + ene come at the end Example: 2-methyl 3-Nonene
3 methyl, 1-butene 4 3 2 1
Alkynes Homologous series of unsaturated hydrocarbons Each member contains at least one C C bond General formula = CnH2n-2
Properties of Alkynes Nonpolar low solubility in H2O fairly low melting and low boiling points More reactive than alkenes : triple bond is site of reactivity
Naming Alkynes Same as alkene just add yne to the end instead
HCCH C2H2 ethyne CHCH H H C C C H H propyne C3H4 CHCCH3 H H H C C C C H H H CHCCH2CH3 1-butyne C4H6 H H H C C C C H H H C4H6 2-butyne CH3CCCH3
3 Homologous Series of HCs Name of Series General Formula Ending Alkanes CnH2n+2 -ane Alkenes CnH2n -ene Alkynes CnH2n-2 -yne
Which compound belongs to the alkene series? A. C2H2 B. C2H4 C. C6H6 D. C6H14 Correct answer = B Alkenes follow the format CnH2n A & C are CnHn , D is CnH2n+2
In which group could the hydrocarbons all belong to the same homologous series? A. C2H2, C2H4, C2H6 B. C2H4, C3H4, C4H8 C. C2H4, C2H6, C3H6 D. C2H4, C3H6, C4H8 Correct answer = D Members of homologous series all have same relationship between atoms Every compound in set D fits the formula CnH2n
Which of the following is a saturated hydrocarbon? A. Ethene B. Ethyne C. Propene D. Propane Correct answer = D all alkanes are saturated