Volumetric Analysis
The study of chemical composition involves identification and estimation of substances. Qualitative analysis identifies substances, while quantitative analysis estimates their constituents using various methods like Volumetry, Gravimetry, and Modern techniques. Non-instrumental methods have advantages like accuracy and simplicity, but also drawbacks like time-consuming procedures. Volumetric analysis includes terms like Titrant, Titration, Indicators, and Equivalence Point. Standards like Primary and Secondary standards are crucial for accurate measurements, necessitating specific requirements for primary standards.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Volumetric Analysis DR.S.B. WATEGAONKAR Assistant Professor, Department of Chemistry, Kisan Veer Mahavidyalaya, Wai
Introduction The study of chemical composition involves the following two steps. Identification of a substance Estimation of its constituents.
Qualitative and Quantitative analysis The identification of a substance is called as qualitative analysis estimation of its constituents is called as quantitative analysis Quantitative analysis: Chemical or non instrumental methods: determination of volume or mass. Volumetry: Volume Gravimetry: Mass Modern or instrumental methods: The instrumental methods are based on the determination of pH, potential, conductance, absorbance, etc. For example, pH metry: pH Potentiometry: Potential Conductometry: Conductance Spectrometry: Absorbance
Advantages and Disadvantages of Non- Instrumental Methods Advantages Procedure is accurate and simple. The equipment needed is cheap. Methods are based on absolute measurements. Special training is not required. Disadvantages Procedure is time consuming. There is lack of versatility. Results are not reliable. Accuracy decreases with decreasing amounts. There is lack of specificity.
VOLUMETRIC ANALYSIS Terms in Volumetry Titrant Titration Titre Indicators Titration error Equivalence Point Visual end point
STANDARDS Primary standards: Whose purity verified before going to use Mass Length Time Wavelength
Secondary standards As the primary standards are not always available for measurement, secondary standards are established. They have the reference of the primary standards. Generally volumetric solutions are known as secondary standards Example: Secondary standard solution of NaOH is prepared by taking approximate concentration of NaOH solution with primary standard solution of Oxalic acid.
STANDARDIZATION Requirements of the primary chemical standards: available at low cost and high purity at least about 99.99%. easily soluble in solvent used and have stoichiometric reactions. stable at normal temperature and non-hygroscopic. possess high molecular weight.
REQUIREMENTS OF VOLUMETRIC ANALYSIS equation for the reaction must be known. The reaction should be stoichiometric The reaction must be quantitative The reaction should be rapid. The reaction should not be reversible. A suitable indicator should be known to determine end point of the reaction. standard solution must be available. There should be a marking There should not be any side reaction.
CONCENTRATION Concentration = Amount of Solute Volume of solution Percent by weight of solute = Wt. of Solute x 100 Wt. of Solution
Molarity Molarity of solution (M) = Total numbers of moles of solute Volume of solution in liters M = n V Unit: M or Molar
Normality Normality (N) = Strength in gms per liter Equivalent wt. of solute OR Weight of the solute in gm Weight of the solute in gm = Number of gm equivalents of solute = Number of gm equivalents of solute Equivalent weight Equivalent weight OR Number of gm equivalent of solute Number of gm equivalent of solute Volume of solution in liter Volume of solution in liter Unit: N or Normal
Molality Molality of solution (m) = Number of moles of solute Number of moles of solute Mass of solvent in kilogram Mass of solvent in kilogram Unit: m or Molal
TYPES OF TITRATIONS Acid base titrations or neutralization titrations. Oxidation-reduction titrations. Precipitation titrations and Complexometric titrations.
ACID BASE OR NEUTRALIZATION TITRATIONS NaOH + HCl NaCl Acid Base Salt Water + H2O HA + H2O H3O++ A- B + H2O BH++ OH- H3O+ + OH- 2 H2O or
Acidimetry and Alkalimetry Acidimetry: Free Bases with Standard Acids CH3COONa + HNO3 NaNO3+ CH3COOH Alkalimetry: Free Acids with Standard Base H3PO4+ NaOH NaH2PO4+ H2O
Neutralization indicators The substance to act as an acid base indicator, it should satisfy the following conditions. It must be either acidic or basic. It must be freely soluble and must be chemically stable. It should not consume appreciable amount of titrating solutions. It should show a marked colour change at a definite pH.
Types of Acid-Base Titration i) Strong acid Vs Strong base: Phenolphthalein NaOH + HCl ii) Weak acid Vs Strong base: Phenolphthalein CH3COOH + NaOH iii) Strong acid Vs Weak base: Methyl orange, NH4OH + HCl NH4Cl + H2O iv) Weak acid Vs Weak base: No simple indicator CH3COOH + NH4OH CH3COONH4+ H2O NaCl + H2O CH3COONa + H2O
IODOMETRIC TITRATION The titration in which iodine is used called as iodometric titration. 2CuSO4+ 4KI I2 + 2Na2S2O3 Indicator : Starch 2CuSO4 I2 2Na2S2O3or Cu I Na2S2O3 1 ml of 1N sodium thiosulphate 0.06354 g of Cu 2CuI + I2+ 2K2SO4 Na2S4O6+ 2NaI
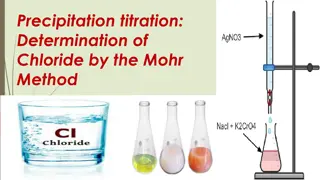
PRECIPITATION TITRATIONS The titration in which ppt is formed called as precipitation titration Cl-+ Ag+ AgCl ( White ppt ) 2 Ag++ CrO4-- Ag2CrO4 ( Brick red ) Indicator: sodium chromate, potassium chromate Burette: Std. AgNO3 Pipette: Water sample contains Cl- ions
COMPLEXOMETRIC TITRATION The titration in which complexing agent is used called as complexometric titration. Burette: Std.EDTA Pipette: hard water sample Indicator: EBT Buffer sample having pH 10.