What is Matter? Explained with Images
The concept of matter through a series of visual slides showcasing its properties, types, changes of state, particle model, temperature, and evaporation. Understand how matter is defined, classified into solids, liquids, and gases, and undergoes transformations like melting, boiling, freezing, and condensing. Discover the behavior of particles, their kinetic energy, and the phenomenon of evaporation. Enhance your knowledge of the fundamental building blocks of the universe in an engaging and informative manner.
Uploaded on Apr 22, 2025 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
What On earth is the MATTER?
A. B. C. ?????
What in the world is matter?
Matter is A. Anything that moves B. Anything that takes up space. C. Something that cries.
Anything that takes up space!
types The Of matter are
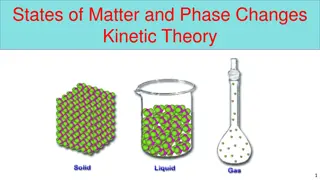
Solids Liquids Gases
How does matter change? Put on your thinking cap!
Changes of state melting boiling solid liquid gas freezing condensing
Changes of state heating curve boiling liquid gas condensing melting solid liquid freezing time
The particle model TIME FOR TIME FOR A SONG A SONG 1. All substances are made up of particles (atoms, ions or molecules). 2. These particles are attracted to each other, some strongly and others weakly. 3. These particles move around (i.e. have kinetic energy). 4. The kinetic energy of particles increases with temperature.
What is evaporation? Evaporation occurs when the particles in a liquid escape to form a vapour. Evaporation can occur at any temperature but it occurs most rapidly at a liquid s boiling point. The particles that escape take some energy from the remaining particles and so the temperature of the liquid falls.