Wilms Tumor - Overview, Risk Factors, and Genetics
Discover the key aspects of Wilms Tumor, the most common pediatric renal cancer. Learn about risk factors, genetic basis, common syndromes associated, and the pathology of this condition. Explore the clinical features, investigations, differential diagnosis, management, and follow-up protocols for Wilms Tumor.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Wilms Tumor/Nephroblastoma Dr Bhojraj Luitel
Learning Objectives Problem statement Etiology/Risk factors Pathology Clinical Features Investigations D/D Management Follow up
Introduction and Problem statement Wilms tumor, or nephroblastoma the most common renal cancer in the pediatric Also the most common pediatric abdominal cancer, and the fourth most common pediatric cancer overall Typically found in children younger than five years old Named after the German physician, Dr. Max Wilms, who first described it in 1899
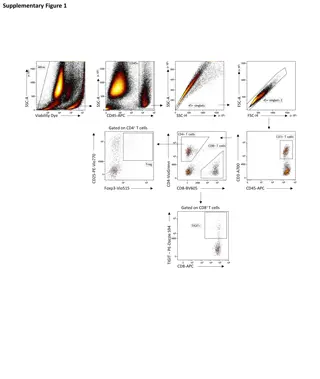
Risk factors and Genetic basis Thought to develop from persistent metanephric tissue or nephrogenic rests These may occur in 1% of infantile kidneys but typically regress during childhood These abnormal metanephric cells are found in up to 100% of cases of bilateral Wilms but only 35% of unilateral tumors
Risk factors and Genetic basis Genetic alterations that deal with the normal embryological development of the genitourinary tract Wilms tumor Some of the genetic mutations that have been associated with Wilms tumor include WT1, CTNNB1, and WTX gene alterations - found in about 1/3 of all Wilms tumors Other genes associated with Wilms tumor include TP53 and MYNC A poorer prognosis - blinked to TP53 and with the loss of heterozygosity at chromosomes 1p, 1q, 11p15 and 16q
Common syndromes associated with Wilms Tumor WAGR Denys Drash Beckwith Wideman syndrome Sotos syndrome, Perlman syndrome, Trisomy 18 (Edward's syndrome), Frasier syndrome, Bloom syndrome, Li-Fraumeni syndrome, and Simpson-Golabi- Behmel syndrome
Pathology of Wilms tumor Triphasic pattern (W-EBS) Epithelial-Blastemal and Stromal Sometimes biphasic The blastema is the most undifferentiated and possibly the most malignant component. It consists of collections of small, round blue cells with very active mitotic activity and overlapping nuclei. FH and UH (favorable and Unfavorable Histology) Based on Absence or presence of Anaplasia
Clinical Features Wilms tumor usually presents as an asymptomatic abdominal mass in the majority of children. The mother may have discovered the mass during bathing the infant. Other features include: Abdominal pain Gross hematuria Urinary tract infections Varicocele Hypertension or hypotension (Up to 1/3 of Wilms patients will demonstrate hypertension which normalizes after nephrectomy) Fever Anemia If the patient has lung metastases, dyspnea or tachypnea may occur. Abdominal pain is the most common initial presenting symptom (30% to 40%) followed by hypertension (25%), and hematuria (12% to 25%).
Investigations: Routine Complete blood count to look for anemia Chemistry profile Renal function Urinalysis Coagulation studies Cytogenetics studies to look for 1p and 16q deletion (LOH)
Investigations: Imagings Renal ultrasonography (often the initial study) but is operator dependent Chest x-ray to look for lung metastases Abdominal and chest CT with sedation Abdominal MRI is an optional study
Staging of Wilms Tumor Stage I - tumor completely contained within the kidney without any breaks or spillage outside the renal capsule and no vascular invasion Stage II - tumor that has grown outside the kidney to some degree, such as into surrounding fatty tissue
Staging of Wilms Tumor Stage III comprises about 20% to 25% of all Wilms tumors and indicates a tumor which could not be completely removed surgically such as the following: Cancer has spread to the regional lymph nodes but not to more distant nodes, such as in the chest Cancer has grown into nearby vital structures so it could not be surgically removed completely Deposits of the tumor (tumor implants) are found in the peritoneum, or there are positive surgical margins Cancer cells were accidentally spilled into the abdominal cavity during surgery The tumor was removed in separate pieces surgically; such as one piece from the kidney and another from the adrenal gland A renal biopsy of the tumor was done before it was surgically removed Stage IV tumors are those that have spread through the vascular system to distant organs such as the lungs, liver, brain, or bones, or to distant lymph nodes Stage V are those cases where both kidneys are involved with tumor at the time of initial diagnosis or Wilms tumor in Solitary kidney Individual staging of each renal unit is needed as well
D/D of Wilms Tumor Clear cell renal Sarcoma Rhabdoid tumor of kidney Neuroblastoma Congenital Mesoblastic nephroma Pediatric RCC Renal medullary carcinoma
Management Surgery: Partial/Radical Nephrectomy Chemotherapy- Neoadjuvant, Adjuvant and Palliative Radiotherapy- to the operative field for stage III tumor and unresectable mets
Management: Two schools of thought National Wilms Tumor Study Group (NWTSG)/Children s Oncology Group (COG) and The International Society of Paediatric Oncology (SIOP) are two major guidelines The major difference exists in the two guidelines is the timing of surgery: SIOP recommends using preoperative chemotherapy, and NWTSG/COG prefers using primary surgery before any adjuvant treatments
Management: Preop Chemotherapy Indications inoperable WT WT in a a solitary kidney synchronous bilateral WT tumor thrombus in the inferior vena cava extending above the level of the hepatic veins tumor involving contiguous structures whereby removing the kidney tumor requiring removal of the other organs, such as spleen, pancreas, or colon extensive pulmonary metastases.
Management: Postop Chemotherapy The COG recommends postoperative chemotherapy routinely used in all patients with WT except those at a very low risk: younger than 2 years at diagnosis with stage I favorable histology tumor weighing <550 gm and negative Lymph node sampling
Tests during Follow Up of a child with Wilms Tumor Abdominal ultrasound (yearly is recommended) CT scan MRI Chest x-ray Liver function testing Renal function blood tests Early screening for infertility Hearing tests for patients who received carboplatin
Follow Up of a child with Wilms Tumor Follow-up visits for Wilms tumor are usually scheduled every 3 months for 2 years after diagnosis then every 6 months for another 2 years then once every 2 years Follow-up visits for Wilms tumor may include: History, clinical exams and Investigations to look for reurrence, mets and complications of treatment
Tests during Follow Up of a child with Wilms Tumor Cardiac function tests, such as an echocardiogram or electrocardiogram at least every 3 years for patients who received doxorubicin (Adriamycin) Early screening for colon cancer in patients who received abdominal radiation (should start 10 years after radiotherapy treatment or by age 35, whichever is later) After lung or chest radiation, the following is added: Thyroid-stimulating hormone (TSH) and free thyroxine (T4), to monitor thyroid function Thyroid US is recommended every 3 years to screen for thyroid nodules and masses Bone density testing should be started 10 years before it would normally be done due to early osteoporosis Early screening for breast cancer in women