Zayed University Information Literacy Program Overview
Zayed University's Information Literacy Program, overseen by Fiona Hunt, is an integral part of the academic curriculum. The program focuses on enhancing students' competencies in information literacy, technological literacy, language proficiency, global awareness, critical thinking, quantitative reasoning, and leadership skills. Through courses like COL 120 and COL 105, students develop research skills, critical thinking abilities, and effective information evaluation techniques. The program also includes library visits, hands-on sessions, and assessments like annotated bibliographies and final exams to ensure students are well-prepared for their academic and professional pursuits.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
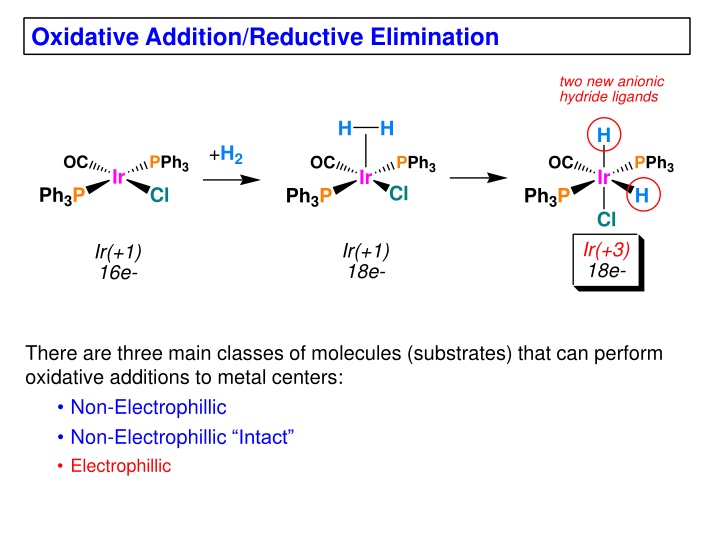
Oxidative Addition/Reductive Elimination two new anionic hydride ligands H H H +H2 OC PPh3 Cl OC PPh3 Cl OC PPh3 H Ir Ir Ir Ph3P Ph3P Ph3P Cl Ir(+3) 18e- Ir(+1) 18e- Ir(+1) 16e- There are three main classes of molecules (substrates) that can perform oxidative additions to metal centers: Non-Electrophillic Non-Electrophillic Intact Electrophillic
Non-electrophillic: these molecules do NOT contain electronegative atoms and/or are not good oxidizing agents. These molecules usually require the presence of an empty orbital on the metal in order for them to pre-coordinate prior to being activated for the oxidative addition rxn. H2, C-H bonds, Si-H bonds, S-H bonds, B-H bonds, N-H bonds, S-S bonds, C-C bonds, etc. H2 is by far the most important for catalytic applications, followed by Si-H bonds, B-H, N-H, and S-H bonds. C-H bond activation and functionalization is very important, but still not practical.
Non-electrophillic Intact: these molecules may or may not contain electronegative atoms, but they do need to have a double or triple bond present. One also needs a metal center with an empty orbital (16e- or lower count) in order to pre-coordinate the ligand before the oxidative addition occurs. Typical intact ligands that can perform an oxidation addition without fragmenting apart are (O2 can also act as an electrophillic substrate): alkenes, alkynes, and O2 metallocyclopropene R R R PMe3 PMe3 Me3P Pt Pt PMe3 PMe3 -PMe3 R
Electrophillic: these molecules do contain electronegative atoms and are good oxidizing agents. They are often considered to be reactive substrates. These molecules do NOT require the presence of an empty orbital (18e- is OK) on the metal center in order to perform the oxidative addition rxn. X2 (X = Cl, Br, I), R-X, Ar-X, H-X, O2, etc. SN2 nucleophillic attack + +CH3Br H Ph3P CO OC PPh3 Cl Br C Ir Ir HH Ph3P Cl PPh3 Ir(+1) 16e- Ir(+1) 16e- two new anionic ligands two new anionic ligands CH3 CH3 +Br OC PPh3 Cl OC PPh3 Cl + Br Ir Ir Ph3P Ph3P Br Ir(+3) 18e- Ir(+3) 16e-
In the case of a starting 18e- complex (shown below) only one of the two anionic ligands (usually the strongest binding) generated from the oxidative addition will end up coordinated to the metal unless a separate substitution reaction occurs. oxidative addition CO H OC H CO OC + Cl + Cl Re OC Re OC OC CO OC CO two new anionic ligands Re(-1) 18e- Re(+1) 18e- -CO CO OC 1) CO ligand dissociation 2) 1- to 3-allyl hapticity change Re OC OC Re(+1) 18e-
WARNING: d0 metals can NOT do oxidative additions!! So always electron count the starting and final metal complexes to check out the overall electron- count, metal oxidation state and d-electron count!
Kinetic Data for Oxidative Addition Reactions of MX(CO)(PR3)2 H S (J/mol K) 23 14 Rate Const (M 1 sec 1) 0.67 10.5 M X Reactant PR3 (kcal/mol) 10.8 12.0 Ir Cl Br PPh3 H2 I > 100 3.4 x 10 2 7.4 x 10 2 21 24 Ir Cl Br 13.1 11.8 PPh3 O2 30 x 10 2 3.5 x 10 3 1.6 x 10 3 0.9 x 10 3 3.5 x 10 2 3.7 x 10 5 12.7 x 10 4 51.5 x 10 4 24 51 46 I 10.9 5.6 7.6 Ir Cl Br CH3I PPh3 43 35 28 44 43 I 8.8 8.8 14.9 9.1 10.2 Ir Cl CH3I P(p-C6H4-OMe)3 P(p-C6H4-Cl)3 PPh3 P(p-C6H4-OMe)3 Rh Cl CH3I Data adapted from Principles and Applications of Organotransition Metal Chemistry , Coleman, Hegedus, Norton & Finke, University Press, 1987; refs: Chock & Halpern, JACS, 1966, 88, 3511; Ugo, Pasini, Fusi, Cenini, JACS, 1972, 94, 7364; Douek & Wilkenson, J. Chem. Soc. (A), 1964, 2604. Rxns generally run in benzene at 25 C.
Oxidative additions are easy to identify IF YOU ELECTRON COUNT the metal complexes. When an oxidative addition rxn occurs the metal will be oxidized, usually by 2e-. So, if you start with a metal in the 0 oxidation state (d8), after the oxidative addition the metal will be in the +2 oxidation state (d6). Once you get used to looking at organometallic rxns you will be able to identify common oxidative additions quite quickly. H2, R-X, and H-SiR3 are three of the most common substrates that perform oxidative addition reactions in catalytic cycles. Problem: H2 will do an oxidative addition most readily to which of the following complexes. Why? b) a) (MeO)3P Cl Me3P Br Ir Rh OC P(OMe)3 OC PMe3 O c) C Pt CO Me3P PMe3
Problem: Cl2 will do an oxidative addition most readily to which of the following complexes. Why? a) b) PMe3 CO OC CO Pd Re PMe3 Me3P OC CO PMe3 CO c) Cl Ti Cl
Problem: CH3Br will do an oxidative addition most readily to which of the following complexes. Why? b) a) PMe3 O Pt Hf NCMe NR2 Me3P Me3P NCMe NR2 c) CO (PhO)3P CO P(OPh)3 Fe OC
Oxidative Coupling Cl Cl Ph2 P Ph2 P H3C N H3C N Cr Cr P Ph2 P Ph2 Cl Cl Cr(+3) d3 15e- Cr(+5) d1 13e- The Cr on the right now has two new anionic alkyl ligands forming a metallocyclopentane ring system. We have done an oxidative addition, but in forming a new bond between the two ethylene ligand (and losing the original double bonds) we have coupled the two ligands together. While this is an oxidative addition, there is a special term for this type of reaction called oxidative coupling. The metal is being oxidized to create two new anionic ligands, but the original two neutral ligands also form a new bond between them, instead of fragmenting apart to make two new independent anionic ligands.
Hydrogenolysis Theonly way that early transition metals with d0 counts can activate H2. Lanthanides and actinides also typically use hydrogenolysis. As with oxidative addition, the metal center needs to have an empty orbital to bind the H2 and an anionic ligand (e.g., alkyl, halide) that can be protonated off. No change in oxidation state of the metal.
Reductive Elimination A reductive elimination reaction is the reverse of an oxidative addition. It is a reaction in which two cisoidal anionic ligands on a metal center couple together. Each anionic ligand pushes one electron back onto the metal center (in the case of a monometallic complex) to reduce it by 2e-. The coupled anionic ligands then usually fall off the metal center as a neutral molecule.
While reductive elimination can occur from saturated 18e- complexes (so long as the two ligands that you want to reductively eliminate are cisoidal to one another), it has been shown that reductive elimination can be promoted by a ligand dissociation generating an unsaturated and more electron-deficient metal center. CH3 CH3 -I Ph2 P Ph2 P Ph2 P CH3 CH3 CH3 + CH3CH3 Pt Pt Pt P Ph2 CH3 P Ph2 CH3 P Ph2 I Pt(+4) 18e- Pt(+4) 16e- Pt(+2) 14e- +I Ph2 P The dissociation of the I- generates a cationic unsaturated complex. This is electron deficient enough to help promote the reductive elimination of ethane (CH3CH3). CH3 Goldberg, J. Am. Chem. Soc. 1995, 117, 6889-6896 Pt P Ph2 I Pt(+2) 16e-
In studying the above system, it was also found that one could have reductive elimination of CH3I from the starting 18e- complex. This reaction, however, is very reversible due to the high reactivity of CH3I for doing an oxidative addition back reaction with the electron-rich neutral Pt(+2) complex to make the Pt(+4) octahedral compound. reductive elimination The reductive elimination of the CH3I is kinetically favored. This is because the orbitals around the iodide anion are spherically symmetric and this makes CH3 Ph2 P Ph2 P CH3 CH3 + CH3I Pt Pt P Ph2 CH3 P Ph2 CH3 it much easier to overlap with the alkyl group orbital to perform the reductive elimination. The sp3 directed orbitals on the two CH3 groups are more difficult to overlap in order to get the reductive elimination to occur. But the reductive elimination of the CH3CH3 is thermo- dynamically considerably more favorable I Pt(+4) 18e- Pt(+2) 16e- oxidative addition and the back oxidative addition much more difficult.
Problem: Which of the following compounds will be most likely to do a reductive elimination of ethane (CH3-CH3)? Why? a) b) NCMe CH3 (MeO)3P CH3 Me3P CH3 Os V H3C P(OMe)3 Me3P CO CO NCMe c) PMe3 MeCN CH3 Pt Ph3P CH3 CH3
Binuclear Systems Complexes with two (or more) adjacent metal atoms can also participate in oxidative addition and reductive elimination reactions. This often involves both metal centers. If so, then each metal changes its oxidation state by only 1, instead of 2 as occurs with single metal centers. This also often involves the making or breaking of a M-M bond, depending on what is present in the starting complex. Cl Cl Cl Au Au Au + Cl2 Me2P PMe2 Me2P PMe2 + Cl2 Me2P PMe2 Au Au Au Cl Cl Cl Au-Au = 2.6 Au-Au = 3.1 Au-Au = 3.0 Au(+2) d9 Au(+3) d8 Au(+1) d10
OO C C h Ru Vollhardt, JACS, 1983, 105, 1676. Ru Ru Ru-Ru = 2.82 Ru CC O C C O C O O C O O Ru(+1) d7 Ru(+2) d6 D3C CD3 D3C CH2 H2C CH2 D Me2N NMe2 O -hydride elimination O O O O O Mo Mo Mo Mo Mo Mo + D3C CH2D Me2N Me2N - CH2=CD2 Me2N Me2N bimetallic reductive elimination O O O O O O NMe2 NMe2 Chisholm & coworkers NMe2 Mo(+3) d3 16e- Mo(+3) d3 16e- Mo(+2) d4 14e-
In general bimetallic reductive eliminations occur across two metals when there is a M-M bond and when one can have good overlap of the two groups orbitals. Notably, there are very few if any examples of two alkyl groups performing a bimetallic reductive elimination.
Problem: Which of the two bimetallic complexes shown below will be most likely to do a reductive elimination of H2? Why? (P = PR3 groups) A B