Understanding Ionic and Covalent Bonding in Chemistry
Ionic bonding involves the transfer of electrons between a metal and a non-metal to form a giant lattice structure, like in sodium chloride and lithium oxide. Covalent bonding, on the other hand, occurs between non-metals, resulting in giant covalent structures or simple molecules. Examples such as magnesium fluoride and magnesium oxide demonstrate how atoms interact to create these different types of bonds.
Understanding Ionic and Covalent Bonding in Chemistry
PowerPoint presentation about 'Understanding Ionic and Covalent Bonding in Chemistry'. This presentation describes the topic on Ionic bonding involves the transfer of electrons between a metal and a non-metal to form a giant lattice structure, like in sodium chloride and lithium oxide. Covalent bonding, on the other hand, occurs between non-metals, resulting in giant covalent structures or simple molecules. Examples such as magnesium fluoride and magnesium oxide demonstrate how atoms interact to create these different types of bonds.. Download this presentation absolutely free.
Presentation Transcript
Ionic bonding (metal + non-metal) Ionic bonds form a giant lattice structure
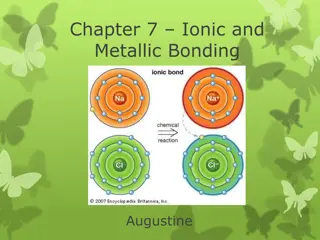
Sodium chloride Sodium chloride Sodium chloride is an ionic compound formed by the reaction between the metal sodium and the non-metal chlorine. sodium Na chlorine Cl sodium chloride NaCl + During the reaction, one electron is transferred from each sodium atom to each chlorine atom.
Sodium chloride Sodium chloride Chlorine has 7 electrons in its outer shell. If it gains 1 electron, it will completely fill its outer shell. Sodium has 1 electron in its outer shell. If it loses this electron, it will have no partially-filled shells. - + Cl Cl Na Na [2.8]+ [2.8.8]- 2.8.1 2.8.7
Sodium chloride Sodium chloride The positive sodium ions and the negative chloride ions are strongly attracted to each other and form an ionic bond. - + Cl Na
Lithium Oxide Lithium Oxide + Li Li 2- O O [2]+ 2.1 + Li Li [2.8]2- 2.6
- Magnesium fluoride Magnesium fluoride F F 2+ [2.8]- 2.7 Mg Mg - F F [2.8]2+ 2.8.2
Magnesium Oxide Explain how magnesium oxide is formed. Magnesium loses 2 electrons Oxygen gains 2 electron Magnesium becomes 2+ ion Oxygen becomes 2- ion Held to together in ionic lattice
Calcium chloride - CaCl2 Explain how CaCl2 is formed: Calcium loses 2 electrons Each chlorine atom gains 1 electron Two chlorine atoms needed Forms ionic bond
Covalent bonding (non-metal + non-metal) Giant covalent structures Simple molecules
Simple molecules HYDROGEN WAYS TO REPRESENT THE MOLECULE H H H H Hydrogen atom needs one electron to complete its outer shell Another hydrogen atom also needs one electron to complete its outer shell H H atoms share a pair of electrons to form a single covalent bond A hydrogen MOLECULE is formed
Simple molecules HYDROGEN CHLORIDE Cl H Hydrogen atom also needs one electron to complete its outer shell Chlorine atom needs one electron to complete its outer shell WAYS TO REPRESENT THE MOLECULE atoms share a pair of electrons to form a single covalent bond H Cl H Cl
Simple molecules AMMONIA WAYS TO REPRESENT THE MOLECULE H N H H H N Each hydrogen atom needs one electron to complete its outer shell H H N H Nitrogen atom needs 3 electrons to complete its outer shell H H Nitrogen can only share 3 of its 5 electrons otherwise it will exceed the maximum of 8 A LONE PAIR REMAINS
Covalent bonding - molecules Oxygen - O2 (g) Hydrogen - H2 (g) Chlorine - Cl2 (g) Hydrogen chloride HCl (g) Methane CH4 (g) Water H2O (l) Ammonia NH3 (g)
Limitations of using models Limitations of using models doesn t show the shape shape doesn t show the
In a metal the atoms LOSE SEVERAL OF THEIR OUTER ELECTRONS which drift around between the metal ions as FREE ELECTRONS. Atoms become POSITIVE ions because they have LOST electrons Free ( delocalised ) electrons
Metallic bonding Metals have a structure of positive metal ionsheld together by a sea of electrons causes electrostatic attraction We call these electrons delocalized Ions are arranged in layers Forms a giant lattice structure
Predicting states using melting and boiling Predicting states using melting and boiling points points Substance Melting Point ( C) Boiling Point ( C) State at room temperature Water 0 99.98 Liquid Carbon Dioxide -78 -57 Gas Methane -182 -164 Gas Hydrogen -259.1 -252.8 Gas Ammonia -77.73 -33.34 Gas Boiling point Melting point Solid Liquid Gas
State Symbols (s) solid (l) liquid (g) gas (aq) dissolved in water NaOH (aq) Na+ (aq) + OH (aq)
Properties of ionic compounds giant lattices High Melting point lots of ENERGY needed to break the strong bonds (strong electrostatic attraction) Solubility - Can dissolve in water which enables the ions to move Conduction - When MOLTEN or DISSOLVED IN WATER, ionic compounds can conduct electricity because the ions can carry current/charge (not electricity)
Covalent bonding simple molecules Properties of covalent compounds Hydrogen - H2 (g) Oxygen - O2 (g) A covalent bond is a shared pair of electrons Chlorine - Cl2 (g) Methane CH4 (g) Substances that consist of simple molecules are gases, liquids or solids that have relatively low melting points and boiling points due to weak intermolecular bonds Hydrogen chloride HCl (g) Ammonia NH3 (g) Water H2O (l) They do not conduct electricity because the molecules do not have an overall electric charge. No free electrons or ions.
Polymers Molecules are linked with strong covalent bonds Intermolecular forces between polymers are relatively strong Polymers are usually solid at room temperature
Giant covalent structure of diamond Carbon structures Carbon structures - - diamond diamond Diamond Diamond is made only from carbon atoms. Every carbon makes four covalent bonds to achieve a full outer shell. Every carbon atom is bonded to four other carbon atoms. This means the structure keeps on growing! We make a Giant Covalent Structure. Key properties Diamond is very hard. High melting points because it has strong covalent bonds (which take a lot of energy to break)