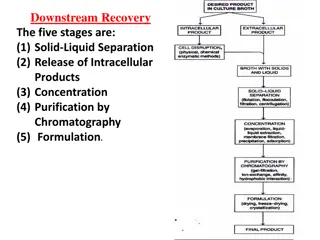
SEPARATION COLUMNS
Azeotropic distillation plays a crucial role in the efficient recovery and recycle of organic solvents in the chemical industry. It addresses the challenges posed by azeotropes in separation processes, offering methods like pressure swing distillation and extractive distillation. Understanding the terminology and different types of azeotropic mixtures is essential for practical industrial applications.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
SEPARATION COLUMNS (DISTILLATION AND ABSORPTION) Dr. Kh. Nasrifar Department of Chemical and Petrochemical Engineering
Importance and industrial relevance of azeotropic distillation Need for efficient recovery and recycle of organic solvents in chemical industry Most liquid mixtures of organic solvents form azeotropes that complicate the synthesis and conceptual design of recovery processes Distillation is the most common unit operation in recovery processes because of its ability to produce high purity products Azeotropes make separation impossible by normal distillation but can be also utilised to separate mixtures not ordinarily separable by normal distillation Azeotropic mixtures may often be effectively separated by distillation by adding a third component, called entrainer Thus, knowledge of the limitations and possibilities in azeotropic distillation is a topic of great practical and industrial interest
Terminology The methods and tools presented in this lecture also appply for: Azeotropic mixtures Close boiling systems Low relative volatility systems Original components A and B: The components that form the azeotrope and need to be separated Entrainer: A third component (E or C) added to enhance separation Binary azeotrope: Azeotrope formed by two components Ternary azeotrope: Azeotrope formed by three components Homogeneous azeotrope: Azeotrope where the forming components are miscible Heterogeneous azeotrope: Azeotrope where the forming components are immiscible
Overview: Azeotropic distillation methods i) Pressure swing distillation ii) Hybrid methods (membrane + distillation) iii) Homogeneous azeotropic (homoazeotropic) distillation iv) Heterogeneous azeotropic (heteroazeotropic) distillation v) Extractive distillation