Acid-Base Titration Process
Learn about acid-base titration, a method to determine the concentration of acids and bases. Explore the procedure, equivalence point, end-point, and solve titration problems with this informative guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
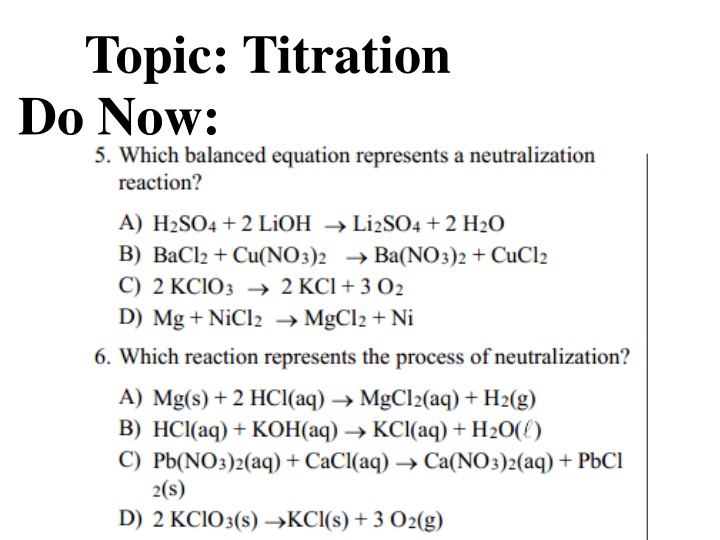
Topic: Titration Do Now:
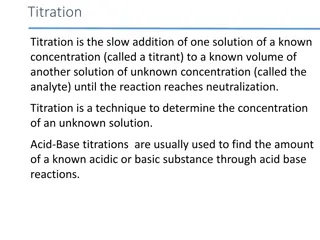
Acid-Base Titration A procedure used in order to determine the molarity of an acid or base Known volume of a solution with a known concentration (standard solution) and known volume of unknown concentration and a MAVA = MBVB acid-base indicator needed
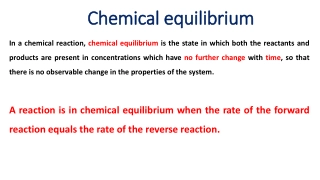
Titration Standard solution slowly added to unknown solution As solutions mix: neutralization reaction occurs Eventually: enough standard solution is added to neutralize the unknown solution Equivalence point
Equivalence point total # moles H+1 ions donated by acid equals total # moles H+1 accepted by base total moles H+1 = total moles OH-1
Titration End-point = point at which indicator changes color if indicator chosen correctly: end-point very close to equivalence point
Titration of a strong acid with a strong base 14- Phenolphthalein Color change: 8.2 to 10 pH Equivalence Pt 7- 0- Volume of 0.100 M NaOH added (ml) 20 ml 0 ml 40m l
MH+1 VH+1= MOH-1 VOH-1 MH+1= molarity of H+1 MOH-1= molarity of OH-1 VH+1= volume of H+1 VOH-1= volume of OH-1
Titration Problem #1 In a titration of 40.0 mL of a nitric acid solution, the end point is reached when 35.0mL of 0.100M NaOH is added Calculate the concentration of the nitric acid solution HNO3 + NaOH H2O + NaNO3
Variables # of H s = 1 Ma = ? Va = 40.0 mL # of OH s = 1 Mb = 0.100 M Vb = 35.0 mL
(1)(X) (40.0 mL) = (0.100 M )(35.0mL)(1) X = 0.875 M HNO3
Titration Problem #2 What is the concentration of a hydrochloric acid solution if 50.0 mL of a 0.250M KOH solution is needed to neutralize 20.0mL of the HCl solution of unknown concentration? KOH + HCl H2O + KCl
Variables # of H s = 1 Ma = X Va = 20.0 mL # of OH s = 1 Mb = 0.250 M Vb = 50.0 mL
(1)(X)(20.0 mL) = (0.250 M) (50.0 mL)(1) X = 0.625 M HCl
Titration Problem #3 What is the concentration of a sulfuric acid solution if 50.0mL of a 0.25 M KOH solution is needed to neutralize 20.0mL of the H2SO4 solution of unknown concentration? H2SO4 + 2 KOH 2 H2O + K2SO4
Variables # of H s = 2 Ma = X Va = 20.0mL # of OH s = 1 Mb = 0.25M Vb = 50.0mL
(2)(X)(20.0ml) = (0.25M)(50.0ml)(1) X = 0.3125 M H2SO4(sulfuric acid)