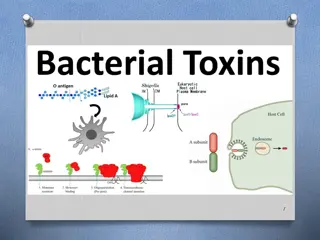
Identifying Toxins Through Molarity Calculations
Explore the use of molarity calculations to detect toxic substances in solutions. Understand how the mass of a solution sample relates to the molar mass of the solute. Dive into discussions on the toxicity of various substances and their molarities. Discover if molarity can be utilized to identify toxins effectively.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Living By Chemistry SECOND EDITION Unit 4: TOXINS Stoichiometry, Solution Chemistry, and Acids and Bases
Lesson 83: Is It Toxic? Mystery Solutions
ChemCatalyst Suppose you wanted to determine if your tap water contains lead (II) nitrate, Pb(NO3)2, which is toxic. 1. Do you expect the mass of 100 mL of pure water to be the same as that of a solution containing Pb(NO3)2? Explain your reasoning. 2. What is the molar mass of lead nitrate? What would be the mass of 0.100 mole of lead (II) nitrate?
Key Question Can molarity calculations be used to identify a toxin?
You will be able to: explain the relationship between the mass of a solution sample and the molar mass of the solute
Prepare for the Classwork Work in pairs.
Discussion Notes The molarities of the 100 mL samples of solute solution were all 1.00 M. To figure out the identities of the solutions, we determined the molar mass of each from the periodic table. It is important to know the toxicity of a substance in moles per kilogram.
Wrap Up Can molarity calculations be used to identify a toxin? Solution samples can have different masses depending on the molar mass of the substance dissolved in the solution. In some situations, it is important to compare numbers of moles of a substance rather than masses.
Check-In You have three aqueous solutions, 200 mL of each: 1.5 M NaCl, 1.0 M KCl, and 1.0 M CaCl2. Which has the greatest mass? Which has the least?