Ionic and Covalent Compound Structures and Properties
Explore the differences between ionic and covalent compounds through their distinct crystal and molecular structures. Discover how these structural differences dictate the properties exhibited by each type of compound, from high melting points and conductivity in ionic compounds to the flexibility and lower melting points of covalent compounds.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
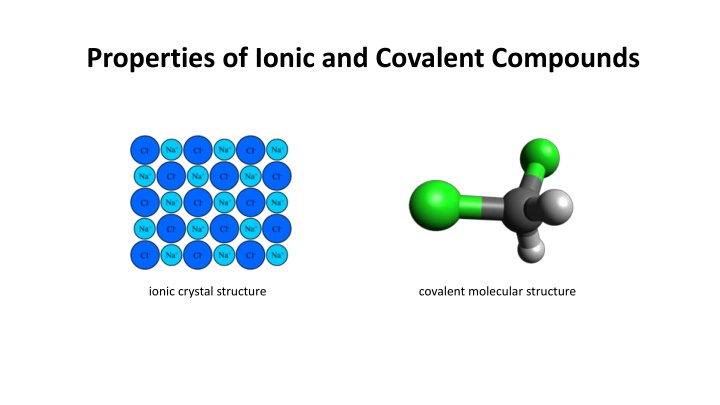
Properties of Ionic and Covalent Compounds ionic crystal structure covalent molecular structure
The structure of compounds determines their The structure of compounds determines their properties properties What a compound looks like on a microscopic level determines how it behaves on a macroscopic level. This is why it s important that we know how ionic compounds and covalent structures differ from one another. Methane is a covalent compound
Structure of Ionic Compounds Structure of Ionic Compounds Ionic compounds form crystal lattices in which one ion is surrounded with many other ions with the opposite charge. These crystals have no theoretical limit to how big they can grow because the addition of additional ions will make the crystal grow larger. A copper (II) sulfate crystal
These rigid structures give rise to the properties of ionic compounds Ionic compounds have high melting and boiling points because it takes a very large amount of energy (i.e. high temperature) to pull all of the cations and anions apart from each other. Ionic compounds are hard because the ions are tightly held in place. Ionic compounds are brittle because any movement of the ions will cause ions with similar charge to come into contact with each other. Ionic compounds conduct electricity when melted or dissolved because electricity can be conducted through moving ions (but not stationary ones). Ionic compounds don t burn because carbon and hydrogen don t generally form ionic compounds.
Covalent compounds, on the other hand, form molecules. Each molecule exists independently of other molecules. There is no covalent bonding between these molecules, so the molecules tend to move around all over the place under normal conditions. Ionic crystals act like LEGO blocks that are stuck rigidly to one another; covalent molecules behave more like Tylenol capsules that can easily move around one another.
Because these molecules dont stick well to each Because these molecules don t stick well to each other, covalent compounds have the following other, covalent compounds have the following properties: properties: Covalent compounds have low melting and boiling points because they melt differently than ionic compounds Ionic compounds melt when the ionic bonds are broken. Covalent compounds melt when the molecules can move away from each other. No bonds are broken, and simply moving them away from each other requires little energy.
Covalent Compounds Are Soft And Squishy Covalent Compounds Are Soft And Squishy OK this might be a bit of an overstatement. After all, ice isn t particularly soft or squishy. However, because covalent molecules don t lock in place next to each other, they re usually not as hard and brittle as ionic compounds because they can deform more easily. Consider rubber (covalent) vs. salt (ionic)
Covalent Compounds Sometimes Catch Fire Covalent Compounds Sometimes Catch Fire Unlike ionic compounds, which hardly ever contain carbon and hydrogen, many covalent compounds do. As a result, many covalent compounds are flammable.
Covalent Compounds Are Usually Less Soluble In Water Than Ionic Compounds Water molecules can easily grab onto the cations and anions in ionic compounds and pull them apart. Covalent compounds don t very often have areas with such strong electric charge that water molecules can grab onto. As a result, water molecules don t do a good job of dissolving covalent compounds.
Covalent Compounds Dont Conduct Electricity Covalent Compounds Don t Conduct Electricity In order to conduct electricity, either electrons need to move from one place or another (as in metals) or ions need to move from one place to another (as in melted/dissolved ionic compounds). Since covalent compounds don t have ions or delocalized bonding, they don t conduct.
In summary: In summary: The properties of both ionic compounds and covalent compounds are determined by their microscopic structure. Ionic compounds tend to be hard, brittle, and have high melting and boiling points because the ions are locked tightly in place. Covalent compounds tend to be less rigid and have lower melting and boiling points because the molecules are easy to separate they don t interact with each other much. This is an enzyme or something. But definitely covalent.