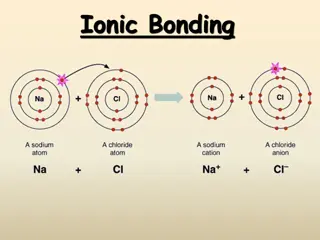
Ionic and Metallic Bonding in Chemistry
Explore the fundamentals of ionic and metallic bonding in chemistry through a series of whiteboard review questions. Learn about the properties of various compounds, including their names, formulas, and conductive abilities in different states of matter. Understand the role of delocalized electrons and why certain compounds exhibit specific characteristics. Dive into the world of chemical bonding with this educational resource.
Uploaded on | 1 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
WHITE BOARD REVIEW
QUESTION What is the name of SO4-2? Sulfate
QUESTION What type of elements are in metallic bonding? How do they bond? Metals to metals with delocalized e-
QUESTION What is the name of CaCl2? Calcium Chloride
QUESTION What is the name of AlP? Aluminum Phosphide
QUESTION What are delocalized electrons? Electrons without a set location
QUESTION In which state of matter can ionic compounds conduct electricity? Liquid (molten) or Aqueous
QUESTION What is the name of the ionic compound Na2O? Sodium Oxide
QUESTION Why are ionic compounds hard but brittle ? High melting points but break easy
QUESTION What is the name of CO3-2? Carbonate
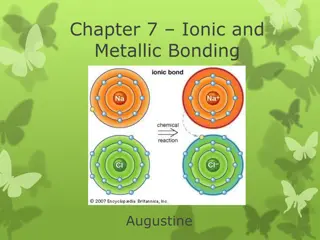
QUESTION Draw the structure of an ionic compound.
QUESTION What is the formula of the ionic compound: Aluminum Sulfide? Al2S3
QUESTION What is an alloy? Mixture of 2 or more metals
QUESTION In which state of matter can ionic compounds NOT conduct electricity? Solid
QUESTION What is the formula for magnesium phosphate? Mg3(PO4)2
QUESTION Why are metallic compounds malleable and ductile? Delocalized electrons allow atoms to slide past each other
QUESTION What is the name of NO3-? Nitrate
QUESTION Name a metallic alloy. Brass, Bronze, Steel, Sterling Silver
QUESTION What is the formula of the ionic compound: Aluminum Oxide? Al2O3
QUESTION Why are alloys important? Superior properties than pure metals and cheaper
QUESTION What type of elements are in ionic bonding? How do they bond? Metal & Nonmetal By transferring e-
QUESTION What is the name of the ionic compound Al2(SO4)3? Aluminum Sulfate