Ionic Liquids for Lanthanides and Actinides Separation Study
Explore the use of ionic liquids as innovative extractants for separating lanthanides and actinides. Discover their utility in recovering critical materials, recycling, and nuclear applications. Learn about the challenges in efficiently separating lanthanides and the potential of ionic liquid extractants. Delve into past work at LANL measuring equilibrium distribution coefficients and developing chromatographic systems for effective separation.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Ionic Liquids as Novel Extractants for Separation of Lanthanides and Actinides Ivis F. Chaple1, Guy Dutech1, Veronika Mocko1, Michael E. Fassbender1* 1Los Alamos National Laboratory, P.O. Box 1663, Los Alamos, NM 87545, USA *Corresponding Author Email Address mifa@lanl.gov Managed by Triad National Security, LLC, for the U.S. Department of Energy s NNSA. 2/28/2022 1 2/28/2022 1
Overview Background Synthesis Data analysis Applications Conclusions Future directions 2/28/2022 2/28/2022 2 2
Background Managed by Triad National Security, LLC, for the U.S. Department of Energy s NNSA. 2/28/2022 3 2/28/2022 3
Background Lanthanides (4f elements) find use in various everyday technologies, while actinides (5f elements) play an important role in nuclear energy applications. Lanthanides are considered critical materials, and the development of lanthanide chemical separation methods is paramount for recovery, recycling and purification purposes. Radiolanthanides also find use as clinical diagnostic (152Tb, 140Nd) and therapeutic (177Lu, 161Tb) agents. Lanthanide separation from each other is difficult due to the nearly identical chemical properties. In order to meet the goal of efficient lanthanide separation, IL extractants will be evaluated for liquid-liquid extraction efficiency. 2/28/2022 4
Ionic Liquids At the most basic level, RT ionic liquids are salts that are liquid at room temperature They are usually composed of large cations, and anions of varying sizes Less optimal sizes of anions and cations in these salts, are the reason ionic liquids, are liquid at room temperature Ionic liquids have been observed throughout history In 1914, [EtNH3][NO3] was synthesized with a melting point of 12 C (53.6 F) [1,2] They can be used to extract metal ions, in place of other more conventional molecular solvents Environmentally friendly. Low toxicity 1. Welton, T. (1999). "Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis." Chemical Reviews 99(8): 2071-2084. 2. Wasserscheid, Peter, and T. Welton. Ionic Liquids in Synthesis. Wiley-VCH, 2008 2/28/2022 5
Cyphosil 104 A phosphonium ionic liquid 2/28/2022 6
Previous work at LANL Measured equilibrium distribution coefficients for Ln(III), Sc(III), Y(III), Th(IV), U(VI), and 225Ac(III) on the SIR from solutions of HNO3 and HCl. Developed chromatographic column systems for the separation of 225Ac from Ln and Ln from each other. Inert solid phase IL solvent Extractant DODGAA Amberlite XAD7HP [Bmim][NTf2] 2/28/2022 7
Equilibrium Distribution Coefficients: Asymmetric Ln Sorption Behavior High selectivity for heavier Ln High separation factors for lighter Ln M.T. Friend et al, Extraction chromatography of 225Ac and lanthanides on N,N-dioctyldiglycolamic acid /1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide solvent impregnated resin, J Chrom A, 1624, 461219. UNCLASSIFIED 2/28/2022 8
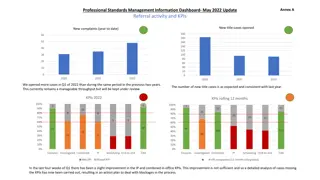
Previous work at LANL Column chromatography: two possible separation systems (there are more: U/Th, Th/REE) 225Ac// 223/224/225Ra/140Ln/141/144Ce 10 M HNO3 4 M HCl 100 80 213Bi 225Ra 225Ac La Ce Pr Nd Elution yield (%) 60 Ac/Ln separation parameters: 1 mL resin bed, 0.1 mL min 1, 5 mg L 1 La-Lu (excluding Pm) each; spiked with 5 kBq of 225Ra(II) and 10 kBq of 225Ac(III). 101 3% 225Ac recovery 40 20 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 140Nd//141Pr Elution volume (mL) 50 10 M HNO3 6 M HNO3 4 M HNO3 3 M HNO3 4 M HCl 0.01 M HF La Gd 40 Ln/Ln separation parameters: 2 mL resin bed, 0.03 mL min 1, 5 mg L 1 each Ln(III). Elution yield (%) 30 Ce Nd Sm 20 Eu Dy Tb Pr 10 Er/Tm/Yb/Lu Ho 0 0 20 40 60 80 100 120 140 160 180 200 220 240 Elution volume (mL) 2/28/2022 9
Synthesis Managed by Triad National Security, LLC, for the U.S. Department of Energy s NNSA. 2/28/2022 10 2/28/2022 10
Resin Synthesis Cyphosil 104 Impregnated Amberlite Peak at 983 cm-1 shifted to 947 cm-1 when functionalized due to stretching vibration of the bond Cr-O Peaks at 2930cm-1, 2859 cm-1, and 2957 cm-1 are stretching vibrations of CH3 and CH2 in Cyphos IL 104 Peak at 1735 cm-1 is the vibration of C=O in resin 0.4 Amberlite Cyphos IL 104 Amberlite + Cyphos IL 104 0.3 * Transmittance * 0.2 * 0.1 0.0 1000 2000 3000 4000 Wavenumber 2/28/2022 11
Data Analysis Managed by Triad National Security, LLC, for the U.S. Department of Energy s NNSA. 2/28/2022 12 2/28/2022 12
Preliminary Results 24 hour Kd Batch Study NH4NO3 Log 24 hour incubation (NH4NO3) 2 hour incubation (HNO3) 7.5 Ce Dy Er Eu Ho La Lu Pr Sm Tb Tm Yb U Th Sc Y 8 Ce Dy Er Eu Gd Ho La Lu Nd Pr Sm Tb Tm Yb U Th Sc Y 7.0 7 6.5 6 Kd Log Kd Log 6.0 5 4 5.5 3 0 1 2 3 4 5 6 7 5.0 HNO3 (M) 0 2 4 6 M NH4NO3 2/28/2022 13
Column Separation 2/28/2022 14
Section Two Conclusion Ionic Liquid impregnated resin can be synthesized Separation of certain metals can be achieved A greener alternative to other extractants 2/28/2022 15
Section Three Managed by Triad National Security, LLC, for the U.S. Department of Energy s NNSA. 2/28/2022 16 2/28/2022 16
Conclusions Radioisotopes find a multitude of uses Current research allows for production, purification, and specific applications in various fields Nuclear medicine is a powerful tool with the potential to impact patient care Ionic liquids may be helpful in the nuclear fuel waste problem 2/28/2022 17
Future Directions Separation of medically relevant radioisotopes Lanthanides; 161Tb, 177Lu. Actinides; 225/227Ac, 227Th. Transition metals; 44/47Sc Radiochemistry Research and development of radiopharmaceuticals 2/28/2022 18
Acknowledgements This research was supported by the U.S. Department of Energy Isotope Program, managed by the Office of Science for Isotope R&D and Production 2/28/2022 2/28/2022 19 19
Thank you! 2/28/2022 20