Lewis Dot Structures and Molecular Polarity
"Explore Lewis Dot structures for single elements and molecules, learn about VSEPR theory, electronegativity, and polarity in compounds. Discover how valence electrons influence the arrangement of atoms and bond polarity in different molecules."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
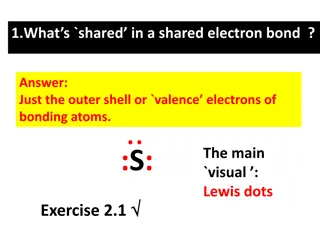
Lewis Dot Structures for Single Elements Ar S Br Li
Mg C B N O K
Molecules H2 H2O
Electronegativity: Important for central atom
PF5 BH3
C2H6 C2H3O21-
CCl4 ICl3
BeCl2 COH2
CClFBr2 SH2
VSEPR valence-shell electron-pair repulsion (abbreviated VSEPR and pronounced VES- per ) theory basic principle is valence electrons around a central atom stay as far apart as possible to minimize the repulsions
Polarity Polarity is the uneven partial charge distribution between various atoms in a compound. Atoms, such as nitrogen, oxygen, and halogens, that are more electronegative have a tendency to have partial negative charges. Atoms, such as carbon and hydrogen, have a tendency to be more neutral or have partial positive charges. Electrons in a polar covalent bond are unequally shared between the two bonded atoms, which results in partial positive and negative charges.
Polarity Polarity Polarity occurs based on the difference in electronegativity between atoms in a molecule having an unequal distribution Electronegativity is an expression of an atom's tendency to attract electrons in a chemical bond In order to determine the polarity of a bond, you must find the difference in the electronegativity of the atoms involved If the difference is between 0.4 and 1.7, the bond will be polar
Polarity Polar

![[PDF⚡READ❤ONLINE] Planet Mercury: From Pale Pink Dot to Dynamic World (Springer](/thumb/21549/pdf-read-online-planet-mercury-from-pale-pink-dot-to-dynamic-world-springer.jpg)