Nanomaterials in NOx Reduction and Catalysis Presentation by S. Sairam
Explore the significance of NOx reduction, catalysts in industrial processes, selective catalytic reduction methods, and the use of titanium dioxide as a support in SCR for effective emission control. Dive deeper into the mechanisms and sources of NOx emissions for a comprehensive understanding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
NANOMATERIALS IN NOX REDUCTION NANOMATERIALS IN NOX REDUCTION PRESENTATION IN NANOMATERIALS IN CATALYSIS BY S.SAIRAM ROLL NUMBER : CH19D023 DEPARTMENT OF CHEMICAL ENGINEERING IIT MADRAS, CHENNAI DATE OF PRESENTATION : 04-03-2020
Why NOx Reduction? Why NOx Reduction? NOX emission (1) Oxides of nitrogen such as N2O, NO and NO2 Sources NOX emission Internal combustion engines(IC) in automobiles Petrol engines and diesel engines(2) Thermal NOX emission(3) High temperature oxidation of N2 in air Natural sources Agricultural fertilization processes and N2 fixing plants(4) Industrial sources of NOX emission(5) https://auto.howstuffworks.com/perce ntage-of-air-pollution-due-to-cars.htm http://www.theozonehole.com/badozone.htm
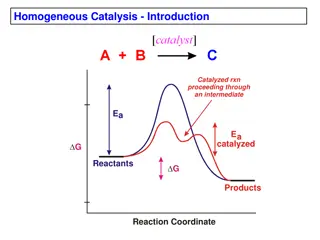
Catalyst in NOX reduction Catalyst in NOX reduction Industrial catalyst in NOX reduction.(5) Metal oxides of base metals on TiO2 V2O5 ,MoO2 & WO3 Mechanism: Selective catalytic reduction of NOX Catalyst in NOX reduction in gasoline engines (6) Stoichiometric fuel/air ratio (3 way catalytic converter) Rhodium: NOX reduction catalyst Melting point of Rh > Exhaust Temp of flu gas High resistance to corrosion- Noble metal Diesel engines Metal Oxides of base metals on TiO2 Mechanism: Selective catalytic reduction of NOX(7) Modifying diesel fuel by blending with additives(8) SCR process in automobiles
Selective Catalytic Reduction Selective Catalytic Reduction Selective Catalytic Reduction Ammonia SCR (9) Diesel engines and lean burn gasoline engines Preferred in industrial NOX emission control Urea-SCR (In automobiles)(9) Lean burn condition under excess oxygen Thermal decomposition to ammonia Safe to store in engine tanks Hydrocarbon-SCR (10) Source :Fuel hydrocarbons No external injection of ammonia Slow NH3 SCR(300-400oC) Fast NH3 SCR reaction (150-300oC) Diesel engines in automobiles Pt/Pd CO +O2 CO2 Reduction of NOX by SCR
Why Titanium Dioxide is used as support in SCR? Why Titanium Dioxide is used as support in SCR? Surface structure of TiO2 (11) Anatase possess large Lewis acidic and Bronsted acidic sites Used in NH3 SCR process Good metal support electronic interactions Good chemical stability Modifies redox property of V2O5 High resistance to sulfur poisoning. Monomeric Vanadyl groups supported on TiO2 support(*) Choo S.T., Lee Y.G., Nam I.S., Ham S.W., Lee J.B. Characteristics of V2O5, supported on sulfated TiO2, for selective catalytic reduction of NO by NH3. Appl. Catal. A Gen. 2000;200:177 188. doi: 10.1016/S0926-860X(00)00636-0. (*) Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. SamiraBagheri, Nurhidayatullaili MuhdJulkapli (11)
V V2 20 05 5 catalysis in NOX reduction catalysis in NOX reduction Commercially important SCR catalyst in industries(12) V2O5/TiO2 catalyst for SCR Possess Bronsted & Lewis acid sites Favors moderate-high temperature NOX reduction. NOX reduction occurs in Bronsted acid site Resistant to SO2 Disadvantages Sintering of catalyst at high temperatures Problem of overoxidation at high temperature Narrow temperature window of operation. (300-400oC) Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration Jie Zhang, Xiangcheng Li , Pingan Chen and Boquan Zhu. (12)
Mechanism of NOX reduction by Mechanism of NOX reduction by - - V V2 2O O5 5 (Ammonia SCR) (Ammonia SCR) NOX reduction occurs in Bronsted acid site in the presence of ammonia Eley-Ridel mechanism of NOX reduction in Bronsted acid site Formation of NH4+ bond in Bronsted acid site (V-OH) Reaction with V=O bond to form intermediate products NO reacts with adsorbed NH3 in the intermediate Decomposition of intermediate products into Nitrogen and water (12)
Nanomaterials in NOx Reduction process Nanomaterials in NOx Reduction process Nanomaterial based catalyst Unique properties Large surface area to volume ratio of nano-catalyst. 10 nm gold SA/Volume : 0.60 (13) 20nm gold SA/Volume : 0.30 Size and shape control facilitates Control of operating temperature (14) Relatively low cost compared to noble metals . Typical nanomaterial in catalysis Metal nanoparticles in catalysis- Gold (15) Nanostructured metal oxides like Mn2O3, CeO2 Nano-gold used in NOX reduction process https://nanocomposix.com/pages/gold-nanoparticles-physical-properties (13) MnO2 Nanowire CeO2 Nanoparticle Composite Catalysts for the Selective Catalytic Reduction of NOx with NH3. Su Hyo Kim, Bum Chul Park, Yoo Sang Jeon, and Young Keun Kim.(14) Gold, an alternative to platinum group metals in automobile catalytic converters. Yanlin Zhang & Robert W. Cattrall & Ian D. McKelvie & Spas D. Kolev.(15)
Ceria nanoparticle- Additive in diesel engines Special properties of Ceria nanoparticle. (16) Excellent redox properties of Ceria Good Ce+3/Ce+4 switching properties. CeO2 CeO2-x+ x/2 O2 Excellent oxygen buffering capacity in nanosized form Oxygen storage capacity in ceria Reduction of NO Ce2O3+ NO 2 CeO2+ N2 Redox activity in ceria is mainly due to Non-stochiometric oxide formation in Ceria. Labile oxygen present in Ceria .(17) Oxidizes CO to CO2and reduces NO to N2 in diesel engines at lower temperatures and promotes complete combustion Oxidation of CO and Hydrocarbons 2CeO2+ CO Ce2O3 + CO2 AjinC.Sajeevan and V.Sajith: Diesel Engine Emission Reduction Using Catalytic Nanoparticles: An Experimental Investigation (16) Structural, redox and catalytic chemistry of ceria based materials. G. Ranga Rao and Braja Gopal Mishra (17)
Synthesis and Characterization of Ceria nanoparticle Synthesis and Characterization of Ceria nanoparticle Nano-Cerium Oxide preparation(16) Precipitation method Cerium Nitrate solution dispersed in isopropanol water mixture with ammonium hydroxide mixture Chemical Reaction: 8Ce(NO3)3+ 22NH4OH +7H2O 8 Ce(OH)4+ 23NH4NO3 Ce(OH)4 + Heat CeO2+ 7H2O Characterization of Ceria nanoparticle Energy dispersive Xray spectroscopy Elemental composition of Cerium & oxygen EDS analysis of Cerium Oxide nanoparticles AjinC.Sajeevan and V.Sajith: Diesel Engine Emission Reduction Using Catalytic Nanoparticles: An Experimental Investigation(16)
Characterization of Ceria Nanoparticles Characterization of Ceria Nanoparticles Cerium oxide nanoparticles in diesel oil Morphology analysis TEM analysis Size reduction of particles Surfactant Xray Diffraction analysis Cubic phase of Cerium oxide crystal . Confirmation of crystallite size(16) Without surfactant With surfactant EDS analysis of Cerium Oxide nanoparticles AjinC.Sajeevan and V.Sajith Diesel Engine Emission Reduction Using Catalytic Nanoparticles: An Experimental Investigation(16)
Effect of Ceria Nanoparticle on NOx Reduction Effect of Ceria Nanoparticle on NOx Reduction Ceria added diesel oil Results of engine performance and emission testing Flash point: Ceria added diesel -Greater flash point than normal diesel. Reduction in volatility of diesel oil NOX emissions control NOX emissions reduced by 30%. Addition of surfactant reduces NOX emission further. Chemical Reaction(16) Ce2O3+ NO 2 CeO2+ N2 Diesel Diesel with 35 ppm CeO2 Diesel with 35 ppm CeO2 and 2% DDSA Diesel with 35 ppm CeO2 and 5% DDSA AjinC.Sajeevan and V.Sajith Diesel Engine Emission Reduction Using Catalytic Nanoparticles: An Experimental Investigation
Promotion of fast SCR Promotion of fast SCR Plasma Fast SCR Reaction Lattice Oxygen Oxidation(MnOx) NO + NO2+ 2NH3 2N2+ 3H2O O + O2 O3 1:1 mole ratio of NO and NO2 Reaction temperature :150 300oC O + NO NO2 Catalyzed by (Plasma-Mn-M)/TiO2 Surface Oxygen Oxidation (18 Plasma- Forms abundant O and O3 radials O + M M Oads Enables oxidation of NO to NO2 NO + M Oads M O NOads Manganese Labile Oxygen M O NOads M + NO2 Favorsl ow temperature NOX Ce/Fe/Co along with Mn- Increases surface oxygen vacancies Mn-O-Fe, Mn-O-Co, Mn-O-Ce. Fe+2 /Ce+2/Co+2 -> M Plasma- -Nano Nano- -bimetallic catalyst bimetallic catalyst O3+ NO NO2 + O2 High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species Haolin Jiang (18)
Mechanism of NOX reduction Mechanism of NOX reduction- -Nano Nano- -bimetallic catalyst bimetallic catalyst Plasma Production of chemisorbed oxygen on catalytic surface (Oads) (18) NO adsorption on catalytic surface NO+Mn+4 NO+ + Mn+3 / Mn+2 Oads + M+2 O- + M+3 NO+ + O- NO2 Fe+2 /Ce+2/Co+2 -> M High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species Haolin Jiang (18)
Catalytic activity Enhancement Catalytic activity Enhancement Fast SCR Fast SCR Effect of surface acidity on ammonia adsorption Temperature controlled desorption of NH3 Temperature controlled desorption to study the strength of adsorption of NH3 on catalytic surface(18) P1: Weak adsorption site, P2&P3 : Medium adsorption site, P4 : Strong adsorption site P1, P2,P3 and P4 shifted to lower temperatures for Mn-Ce/ TiO2 NH3 desorption is high for Mn-Ce/ TiO2 as comparted to Mn-Co/ TiO2 and Mn-Ce/ TiO2 Mn-Ce/ TiO2 possess strong adsorption acid site than Mn-Co/ TiO2 and Mn-Ce/ TiO2 High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species Haolin Jiang (18)
Catalytic activity Catalytic activity Oxidation state analysis Oxidation state analysis XPS Analysis - Determination of Binding Energy and Atomic Composition Mn-Ce/TiO2 exhibited higher Mn+4/Mn+3 ratio as compared to Mn-Co/TiO2 and Mn-Fe/TiO2. O - Chemisorbed Oxygen Binding Energy Mn-Ce/TiO2 < Mn-Co/TiO2 < Mn-Fe/TiO2 O - Lattice Oxygen Binding Energy Mn-Ce/TiO2 < Mn-Co/TiO2 < Mn-Fe/TiO2 Surface chemisorbed Oxygen concentration is more for Ce than Co and Fe(18). High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species Haolin Jiang (18)
Catalytic conversion of NOX Catalytic conversion of NOX- - Nanobimetallic Mn based bimetallic catalyst Mn-Fe/TiO2 catalyst (Minimum catalytic activity) Mn-Co/TiO2 catalyst Mn-Ce/TiO2 catalyst (Maximum catalytic activity) Higher catalytic activity than mono metallic Enhancement of Eley- Rideal mechanism Stronger Mn-O-X X(Fe,Co,Ce) Synthesis route Hydrothermal process Higher NOX conversion at higher temperatures Temperature range-(175-2500C) (18) Nanobimetallic catalyst catalyst Effect of bimetallic catalyst in NOX reduction process High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species Haolin Jiang (18)
Catalytic NOX reduction Catalytic NOX reduction- -Plasma Effect Plasma Effect Catalytic activity-Hybrid systems Bimetallic Mn Nano-catalyst & plasma Increased conversion of NOX levels High catalytic activity than Bimetallic catalyst Plasma only catalyst Acceleration of SCR reaction(18) Oxygen free radicals Nitrogen free radicals Effect of hybrid catalyst- Plasma-Bimetallic catalyst NOX concentration on surface High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species Haolin Jiang (18)
2 Dimensional Mn Metal Oxide Nanoparticle 2 Dimensional Mn Metal Oxide Nanoparticle Manganese based catalyst Large amount of free oxygen on surface. Promotes low temperature SCR. 2 D Mn Metal oxide nanoparticle Higher active sites Large specific surface area. Mn Al-Layered Double Oxide(19) Large Oxygen vacancies Promotion of catalytic activity Mn Al-Layered Double Oxide nanoparticle Enhanced Oxygen Vacancies in a Two-Dimensional MnAl-Layered Double Oxide Prepared via Flash Nanoprecipitation Offers High Selective Catalytic Reduction of NOx with NH3. Dan Zhao 1, Chao Wang 1, Feng Yu 1, ID , Yulin Shi 1, Peng Cao 1, Jianming Dan 1, Kai Chen 1,2, Yin Lv 1, Xuhong Guo 1,2, and Bin Dai 1. (19)
CONCLUSION CONCLUSION Investigated the causes of NOX pollution Analysed the various catalytic reduction processes and catalyst in NOX reduction process. Investigated the role of nano catalyst in NOX pollution abatement. Role of Ceria nanoparticles in NOX abatement.(Preparation, Mechanism and NOX reduction efficiency). Role of Mn-based bimetallic catalyst in NOX reduction process. ( Preparation, Mechanism and NOX reduction process)
FUTURE WORK FUTURE WORK Investigate the preparation and characterization of 2DMn Al-Layered Double Oxide Investigate the effect of various synthesis method on catalytic properties of 2D Mn-Al layered double oxide. Compare the catalytic activity with other Manganese based nanocatalyst used in NOX reduction process.
REFERENCES 1) Mollenhauer, Klaus; Tsch ke, Helmut (2010). Handbook of Diesel Engines. Springer. pp. 445 446. ISBN 978-3540890829. (1) 2) Omidvarborna; et al. (December 2015). "NOx emissions from low-temperature combustion of biodiesel made of various feedstocks and blends". Fuel Processing Technology. 140: 113 118. doi:10.1016/j.fuproc.2015.08.031. (2) 3)Milton R. Beychok (March 1973). "NOX emission from fuel combustion controlled". Oil & Gas Journal: 53 56. (3) 4)J.N. Galloway; et al. (September 2004). "Nitrogen cycles: past, present, and future". Biogeochemistry. 70 (2): 153 226. doi:10.1007/s10533-004-0370-0(4) 5) Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration. Jie Zhang, Xiangcheng Li & Pingan Chen & Boquan Zhu . 6) A. Bera, and M. S. Hegde, Recent advances in auto exhaust catalysis , Journal of the Indian Institute of Science, vol. 90:2, pp.299-325, 2010 Catalyst Platinum and Rhodium.
REFERENCES 7)Passive SCR for lean gasoline NOX control: Engine-based strategies to minimize fuel penalty associated with catalytic NH3 generation. 8) M. G r , U. Karakaya, D. Altiparmak, and A. Alicilar, Improvement of Diesel fuel properties by using additives, Energy Conversion and Management, vol. 43, no. 8, pp. 1021 1025, 2002. 9) NOx Selective Catalytic Reduction (NOx-SCR) by Urea: Evidence of the Reactivity of HNCO, Including a Specific Reaction Pathway for NOx Reduction Involving NO + NO2. M. Seneque, F. Can,* D. Duprez, and X. Courtois 10) Catalysts for NOX selective catalytic reduction by hydrocarbons. (HC-SCR) RayaMrad, Aissa Aissata, RenaudCousin, DominiqueCourcota, St phane Siffert.
REFERENCES 11) Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. SamiraBagheri, Nurhidayatullaili MuhdJulkapli, 12) Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration Jie Zhang, Xiangcheng Li , Pingan Chen and Boquan Zhu. 13) https://nanocomposix.com/pages/gold-nanoparticles-physical-properties 14) MnO2 Nanowire CeO2 Nanoparticle Composite Catalysts for the Selective Catalytic Reduction of NOx with NH3. Su Hyo Kim, Bum Chul Park, Yoo Sang Jeon, and Young Keun Kim. 15) Gold, an alternative to platinum group metals in automobile catalytic converters. Yanlin Zhang & Robert W. Cattrall & Ian D. McKelvie & Spas D. Kolev. 16) Diesel Engine Emission Reduction Using Catalytic Nanoparticles: An Experimental Investigation 17) Structural, redox and catalytic chemistry of ceria based materials. G. Ranga Rao and Braja Gopal Mishra
REFERENCES 18) High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species. Yan Gao, Wenchao Jiang , Tao Luan , Hui Li , Wenke Zhang , Wenchen Feng and Haolin Jiang 19) Enhanced Oxygen Vacancies in a Two-Dimensional MnAl-Layered Double Oxide Prepared via Flash Nanoprecipitation Offers High Selective Catalytic Reduction of NOx with NH3. Dan Zhao 1, Chao Wang 1, Feng Yu 1, ID , Yulin Shi 1, Peng Cao 1, Jianming Dan 1, Kai Chen 1,2, Yin Lv 1, Xuhong Guo 1,2, and Bin Dai 1. Effect of Hydrocarbon on DeNOx Performance of Selective Catalytic Reduction by a Combined Reductant over Cu-Containing Zeolite Catalysts. Iljeong Heo, Samkyung Sung, Min Bum Park, Tae Sun Chang, Young Jin Kim, Byong K. Cho, Suk Bong Hong, Jin Woo Choung In-Sik Nam,