Oxidation States and Ionic Bonding Concepts
Explore the concepts of oxidation states and ionic bonding through the Criss-Cross Method. Learn how to identify if a compound is ionic, covalent, or metallic by analyzing its components. Discover the rules for assigning oxidation states and practice using the criss-cross method to write chemical formulas.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
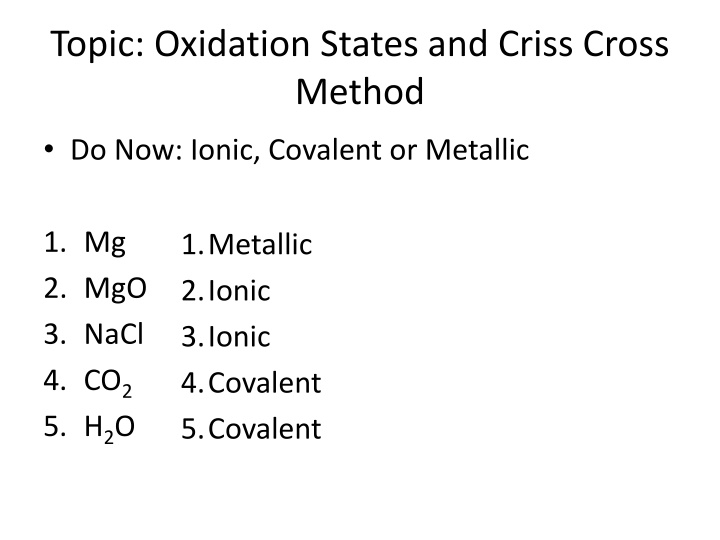
Topic: Oxidation States and Criss Cross Method Do Now: Ionic, Covalent or Metallic 1. Mg 2. MgO 3. NaCl 4. CO2 5. H2O 1.Metallic 2.Ionic 3.Ionic 4.Covalent 5.Covalent
Ionic bond is a bond between: positive ion and negative ion Metals: lose valence electrons form cation (+ ion) Nonmetals: gain electrons form anion (- ion)
Metals lose their valence electrons and nonmetals gain
IONIC BONDING Ionic compounds are electrically neutral overall 1 Ca = +2 x 1 = +2 2 Cl = -1 x 2 = -2 2 K = +1 x 2 = +2 1 S = -2 x 1 = -2 2 Cr = +3 x 2 = +6 3 O = -2 x 3 = -6
Assigning Oxidation States You can look at your periodic table. It s listed in the topic right corner. What do you do if there is more then one?!
What to do if more then 1 oxidation state V2S5 Nonmetal = top number So S would be -2 -2 +5 V2S5 -2 x 5 = -10 Then determine the oxidation state of the metal that will give you the same number but positive ____ x 2 = +10 +5
You try FeO Fe=____ O= ____ +2 -2 -2 Fe2O3 Fe=____ O= ____ +3
Criss-Cross Method Mg+2 and Cl-1 Crisscross and Drop Mg1Cl2 but if subscript is 1, forget it! MgCl2 means 1 Mg+2 and 2 Cl-1
NOTE: The formula has to be in the simplest whole number ratio (this is called the empirical formular Be2O2 = BeO Al3N3 = AlN
Try a few formulas: Ca+2 + Cl-1 Na+1 + O-2 Cs+1 + S-2 Al+3 + Cl-1 Al+3 + Se-2 Mg+2 + F-1 Ca+2 + S-2 CaCl2 Na2O Cs2S AlCl3 Al2Se3 MgF2 CaS