Redox Reactions and Species
Explore the importance of redox pivots, balancing reactions with O2 and H2, and changing the redox pivot in a flexible manner. Learn how databases treat redox reactions and the significance of species like O2(aq) and H2(aq).
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
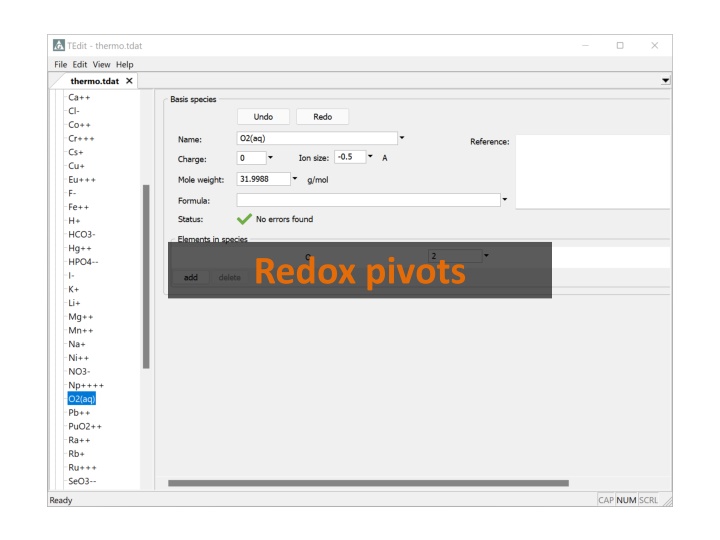
Databases treating redox reactions must include O2(aq) or H2(aq) in the list of Basis Species. This species is the redox pivot . O2(aq) is the redox pivot in thermo.tdat
The reaction for the free electron is written in terms of O2(aq) or H2(aq), or their gaseous forms. Here the electron is balanced in terms of O2(g)
Redox coupling reactions can be balanced in a flexible manner. Balance in terms of O2(aq), O2(g), H2(aq), H2(g), or the e Fe+++, H2(aq), etc. are redox species
You can change the redox pivot by exchanging O2(aq) with H2(aq). Exchange the positions of O2(aq) and H2(aq) After the exchange, reaction is balanced with H2(aq), Fe2+, and H+.