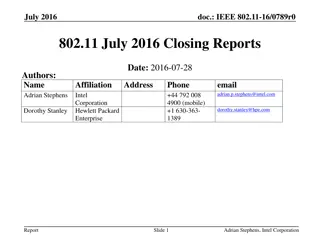
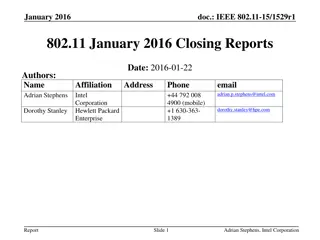
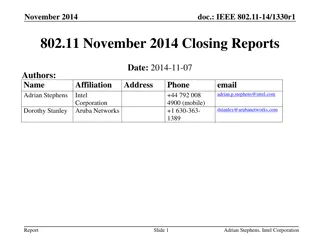
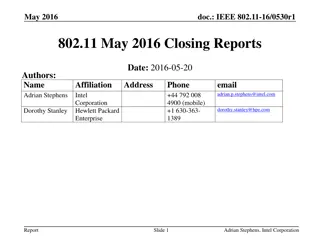
September 2016 IEEE 802.11 Closing Reports
This document provides a digest of the closing reports of various IEEE 802.11 sub-groups presented at the September 2016 closing plenary meeting. It includes attendance information, liaison reports, new members, and more.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Switch to D/C/F/TAF EMERALD Study
EMERALD Study: Switch to D/C/F/TAF Design Randomisation * 2 : 1 Open label W48 W96 HIV+ 18 years On F/TDF + PI/r or/c 6 months Absence of history of virologic failure on DRV (previous VF allowed) Absence of DRV RAM (if historical genotype available) HIV RNA < 50 c/mL 2 months (1 blip 50-200 c/mL within past 12 months allowed) eGFR (Cockroft-Gault) 50 mL/min N = 763 D/C/F/TAF QD D/C/F/TAF Continuation PI/r or /C + F/TDF D/C/F/TAF N = 378 * Randomisation stratified by boosted PI Primary endpoint Proportion of patients with virologic rebound at W48 ; non-inferiority if lower margin of a two-sided 95% CI for the adjusted difference = - 4%, 89% power Virologic rebound: confirmed HIV RNA 50 c/mL (or single HIV RNA > 50 c/mL at W48), or premature discontinuation, irrespective of reason, with last HIV RNA 50 c/mL through W48 Orkin C. Lancet HIV. 2018; 5 :e23-34.; Eron J, IDWeek 2018, Abs. 1768 EMERALD
EMERALD Study: Switch to D/C/F/TAF Baseline characteristics and patient disposition D/C/F/TAF N = 763 46 18.3 75 / 20 / 5 630 41.6 59 15.2 104.2 Continuation PI/r or/C + F/TDF N = 378 45 17.2 75 / 22 / 4 624 42.6 57 14.1 103.3 Median age, years Female, % Race: white / black / other, % CD4/mm3, median On first ARV regimen, % Prior exposure to 5 ARV, % Prior virologic failure, % eGR (Cockroft-Gault), mL/min, median Boosted PI at screening, % DRV / ATV / LPV COBI Discontinuation by W48, N (%) For adverse event, N Withdrew consent, N Lost to follow-up, N Non-compliant, N Other reasons, N 70.8 / 21.5 / 7.7 14.0 34 (4.5) 11 10 5 2 6 70.4 / 21.7 / 7.9 17.2 20 (5.3) 8 5 0 0 3 Orkin C. Lancet HIV. 2018; 5 :e23-34. EMERALD
EMERALD Study: Switch to D/C/F/TAF Cumulative confirmed virologic rebound (HIV RNA 50 c/mL) through W48 Virologic response at W48 (HIV RNA < 50 c/mL), ITT snapshot % % 94.9 93.7 100 100 D/C/F/TAF (N = 763) Continuation PI (N = 378) 80 80 60 60 40 40 Difference : 0.4% (95% CI : - 1.5 to 2.2) 20 20 5.8 4.3 2.5 0.8 2.1 0.5 0 0 HIV RNA < 50 c/mL Difference : 1.2% (95 % CI : - 1.7 to 4.1) HIV RNA 50 c/mL No virologic data 4 patients with virologic rebound genotyped: 1 in D/C/F/TAF (presence of D67D/N) and 3 in continuation group (E138E/G NNRTI mutation in 1) Orkin C. Lancet HIV. 2018; 5 :e23-34 EMERALD
EMERALD Study: Switch to D/C/F/TAF Virologic rebound rate through W48 according to previous antiretroviral failure No previous ARV failure 1 previous ARV failure % % 100 100 D/C/F/TAF (N = 116) D/C/F/TAF (N = 647) Continuation PI (N = 53) Continuation PI (N = 325) 80 80 60 60 40 40 Difference: 0% (95% CI: - 2.6 to 2.0) Difference: 2.6% (95% CI: - 4.8 to 7.5) 20 20 2.5 2.5 2.6 0 0 0 Orkin C. Lancet HIV. 2018; 5 :e23-34. EMERALD
EMERALD Study: Switch to D/C/F/TAF Confirmed virologic rebound (HIV RNA 50 c/mL) HIV RNA < 50 c/mL at W96, ITT snapshot D/C/F/TAF immediate switch D0-W48 (N = 763) D/C/F/TAF immediate switch D0-W96 (N = 763) % % 94.9 100 100 90.7 80 80 60 60 40 40 20 20 3.1 2.5 0 0 Most rebounders (14/24 D/C/F/TAF immediate switch and 2/8 deferrred switch) resuppressed to HIV RNA < 50 c/mL by W96, without treatment change Genotype performed if virologic rebound 400 c/mL (4 D/C/F/TAF immediate switch and 5 deferred switch): no emergence resistance to NRTI or PI Eron J, IDWeek 2018, Abs. 1768
EMERALD Study: Switch to D/C/F/TAF Mean (SE) % change from baseline in bone mineral density (g/cm2) Continuation PI D/C/F/TAF Hip Lumbar spine 2 2 1.49 (3.34) 1.43 (2.34) 1 1 p < 0.0001 p < 0.0001 p < 0.0001 p < 0.0001 0 0 - 0.26 (2.00) - 0.63 (2.86) -1 -1 0 24 48 0 48 24 Weeks Weeks No of participants No of participants D/C/F/TAF Continuation PI 204 104 184 193 188 97 D/C/F/TAF Continuation PI 206 107 192 97 192 101 Lumbar BMD change at W48 D/C/F/TAF Continuation PI Hip BMD change at W48 D/C/F/TAF Continuation PI 31.8 8.9 3% 20.2 4.1 3% 15.1 1.0 5% 4.8 1.0 5% 7.8 19.8 3% 2.1 8.2 3% 1.6 8.9 5% 0 2.1 5% Orkin C. Lancet HIV. 2018; 5 :e23-34 EMERALD
EMERALD Study: Switch to D/C/F/TAF Mean (SE) % change from baseline in bone biomarkers Procollagen type N-terminal propeptide Alkaline phosphatase C-type collagen sequence N = 193 N = 191 N = 185 N = 103 N = 98 N = 98 5 5 20 0 0 10 p < 0.0001 p < 0.0001 p < 0.0001 p < 0.0001 p < 0.0001 -5 -10 0 p = 0.0003 -10 -10 -20 -15 -20 -30 -20 0 24 12 24 48 0 24 12 24 48 0 24 12 24 48 Weeks Weeks Weeks Parathyroid hormone N = 186 25-hydroxy vitamin D N = 96 N = 154 N = 76 20 D/C/F/TAF Continuation PI 30 15 10 20 p = 0.074 p = 0.0019 5 p = 0.495 p = 0.0074 10 0 0 -5 -5 -10 -10 0 24 48 0 24 48 Weeks Weeks Orkin C. Lancet HIV. 2018; 5 :e23-34. EMERALD
EMERALD Study: Switch to D/C/F/TAF Median lipid values TC:HDL-C ratio D/C/F/TAF baseline D/C/F/TAF W48 Continuation PI baseline Continuation PI W48 mmol/L mg/dL 205 * 5.2 3.84 ** 3.83.9 ** 200 181186 184 4 3.9 150 3 123129123128 123 * 103106 107 2.6 100 2 4750 * 48 47 1.3 50 1 0 0 0 * p < 0.0001 W48 vs baseline ; **p < 0.001 D/C/F/TAF vs continuation PI Orkin C. Lancet HIV. 2018; 5 :e23-34 EMERALD
EMERALD Study: Switch to D/C/F/TAF Mean Changes in eGFR (mL/min/1.73 m2) at W48 Between-group comparison p D/C/F/TAF Continuation PI eGFRcyst - 0.4 9.6 - 1.9 10.7 0.034 eGFR cr - 1.9 - 0.9 0.092 Orkin C. Lancet HIV. 2018; 5 :e23-34 EMERALD
EMERALD Study: Switch to D/C/F/TAF Adverse events between D0 and W48, % D/C/F/TAF (N = 763) PI/r or PI/C + F/TDF (N = 378) Grade 3-4 adverse event 7 8 Serious adverse event 5 5 Discontinuation for adverse event Due to renal event 1.4% (N = 11) 0.1% (N = 1 *) 1.3% (N = 5) 0.5% (N = 2 **) Adverse event in 5% of either group Naso-pharyngitis Upper respiratory tract infection Diarrhea Headache Back pain Vitamin D deficiency Osteopenia 11 11 8 8 7 7 5 10 10 4 4 6 7 6 Grade 3-4 laboratory abnormalities LDL-cholesterol 4,9 mmol/L Total-cholesterol 7.77 mmol/L Phosphate < 0,65 mmol/L Total bilirubin 2,6 x ULN 7 4 3 2 1 5 6 < 1 * Worsening of pre-existing renal insufficiency ; ** Toxic nephropathy, N = 1 ; tubulopathy, N = 1 Orkin C. Lancet HIV. 2018; 5 :e23-34 EMERALD
EMERALD Study: Switch to D/C/F/TAF Adverse events, D0-W96(%) D/C/F/TAF (N = 763) PI/b + F/TDF (N = 378) D/C/F/TAF (N = 352) D0-W48 D0-W96 D0-W52 W52-W96 Grade 3-4 adverse events 7.1 12.8 8.2 7.4 Serious adverse events 4.6 8.7 4.8 6.0 Discontinuation for adverse event 1.6 2.2 1.3 2.0 Deaths 0 0.4 * 0 0 * 1 metastatic pancreas cancer and 2 myocardial infarction Eron J, IDWeek 2018, Abs. 1768
EMERALD Study: Switch to D/C/F/TAF Changes in renal, lipid and bone parameters at W96 (%) PI/b + F/TDF (N = 378) D/C/F/TAF (N = 763) D/C/F/TAF (N = 352) D0-W96 D0-W52 W52-W96 Median change in eGDRCKD-EPI, mL/min/1,73 m2 UPCR UACR RBP:CR B2M:CR - 1.3 - 22.23 - 0.63 - 25.08 - 68.22 - 0.6 - 7.37 0.40 + 19.66 + 20.24 - 0.7 - 12.81 - 0.93 - 39.07 - 110.31 Median change in lipids, mg/dL Total cholesterol LDL-cholesterol HDL-cholesterol Triglycerides Total cholesterol/HDL-cholesterol ratio + 22.0 + 17.0 + 3.0 + 7.0 + 0.2 + 1.3 + 1.9 0 + 4.9 + 0.1 + 22.0 + 15.0 + 3.3 + 8.0 + 0.2 Mean % change in BMD Lumbar spine Total hip Femoral neck + 1.99 + 1.86 + 1.40 - 0.63 - 0.27 - 0.51 + 2.91 + 1.22 + 0.98 Eron J, IDWeek 2018, Abs. 1768
EMERALD Study: Switch to D/C/F/TAF Conclusion Through Week 96, switching from boosted PI + FTC/TDF to D/C/F/TAF resulted in: Low virologic rebound rate cumulative (2.5% at W48, 3.1 % at W96) High virologic suppression rate (94.9% at W48, 91% at W96) Virologic non-response rate of 1% No resistance to any study drug Few serious adverse events and discontinuations due to adverse events (2%) D/C/F/TAF bone, renal and lipid safety were consistent with known profiles of TAF and cobicistat Orkin C. Lancet HIV. 2018; 5 :e23-34; Eron J, IDWeek 2018, Abs. 1768 EMERALD