Transition Metals in Catalysis: Importance and Examples
Transition metals play a crucial role in catalysis due to their variable oxidation states. Learn about heterogeneous catalysts, catalyst poisoning, and the support medium in catalysis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
TRANSITION METALS - CATALYSIS Learning Objectives: 1. Describe the importance of variable oxidation states in catalysis. 2. Explain with the aid of equations, how V2O5 acts as a heterogeneous catalyst in the contact process. 3. Explain with the aid of equations, how Fe2+ ions catalyse the reaction between I- and S2O82-, and how Mn2+ ions autocatalyse the reaction between C2O42- and MnO4-. Specification Reference: 3.2.5
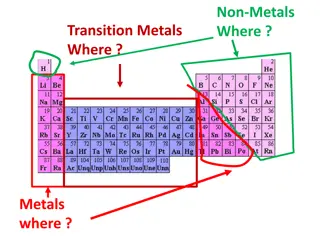
WHY DO TRANSITION METALS MAKE GOOD CATALYSTS? Transition metals make good catalysts because they can change oxidation states by gaining or losing electrons within their d orbitals - This means that they can transfer electrons to speed up reactions. An example of their use is the manufacture of Sulphuric acid in the Contact process: Firstly, vanadium (V) oxide oxidises SO2 to SO3 and is itself reduced to Vanadium (IV) oxide: The reduced catalyst is then oxidised by oxygen back to its original state: If vanadium did not have variable oxidation states, this reaction would not be possible. LO1: Describe the importance of variable oxidation states in catalysis.
HETEROGENEOUS CATALYSTS A heterogeneous catalyst is one that is in a different phase from the reactants i.e. in a different physical state. You need to remember two examples: 1) Iron is used in the Haber process for making ammonia: 2) Vanadium (V) oxide is used in the Contact process: In all of these reactions, the catalyst is a solid and the reactants are gases. LO2: Explain with the aid of equations, how V2O5 acts as a heterogeneous catalyst in the contact process.
HETEROGENEOUS CATALYSTS USE OF A SUPPORT MEDIUM In heterogeneous catalysis, the reaction happens on the surface of the catalyst. Increasing the surface area of the catalyst increases the number of molecules that can react at the same time, increasing the rate of reaction. A support medium is often used to make the area of the catalyst as large as possible.
HETEROGENEOUS CATALYSTS CATALYST POISONING In a chemical reaction, reactants are adsorbed onto the surface of heterogeneous catalysts. Impurities in the reaction mixture may also bind to the catalyst s surface and block reactants from being adsorbed. THE CATALYST HAS BECOME POISONED. Reduced surface area Lower rate of reaction Increased production costs due to lower yield Catalyst needs replacing/regenerating Common examples of catalyst poisoning: Leaded petrol used by mistake in unleaded vehicles produces lead vapour in the exhaust gases that coats the catalyst surface leading to a car failing its emissions test in an MOT. Sulphur poisons iron catalysts in the Haber process when present as an impurity from the synthesis of hydrogen gas from methane.
HOMOGENEOUS CATALYSTS Are in the same physical state as the reactants usually aqueous reactions! These form an intermediate species, which is a combination of the reactants. This then goes on to react further to produce the product. An enthalpy profile diagram contains two humps, representing both stages in the catalysed reaction: LO3: Explain with the aid of equations, how Fe2+ ions catalyse the reaction between I- and S2O82-, and how Mn2+ ions autocatalyse the reaction between C2O42- and MnO4-.
HETEROGENEOUS CATALYSTS - THE IODINE CLOCK REACTION Fe2+ catalysing the reaction between S2O82- and I- The redox reaction between iodide ions and peroxodisulphate (S2O82-) is given below: Why do you think this reaction happens so slowly? This is solved by using an iron (II) catalyst which is oxidised to iron (III) by S2O82- ions: The iron (III) is an intermediate as it then oxidises the iodide ions to iodine, regenerating the catalyst: Iodine can be tested for by using a starch solution What do you observe? LO3: Explain with the aid of equations, how Fe2+ ions catalyse the reaction between I- and S2O82-, and how Mn2+ ions autocatalyse the reaction between C2O42- and MnO4-.
HETEROGENEOUS CATALYSTS - AUTOCATALYSIS Mn2+ catalysing the reaction between MnO4- and C2O42- This is an autocatalysis reaction since the Mn2+ is a product of the reaction which also acts as a catalyst for the reaction. This means that as the amount of product increases, the reaction gets faster! The initial reaction is very slow due to the reactants both being negatively charged ions, however once a small amount of the Mn2+ is produced, it reacts via an alternative route: The Mn3+ are the intermediate. These then react with C2O42- ions to make CO2 and reform the Mn2+ catalyst: LO3: Explain with the aid of equations, how Fe2+ ions catalyse the reaction between I- and S2O82-, and how Mn2+ ions autocatalyse the reaction between C2O42- and MnO4-.
HETEROGENEOUS CATALYSTS - AUTOCATALYSIS Mn2+ catalysing the reaction between MnO4- and C2O42- This is an autocatalysis reaction since the Mn2+ is a product of the reaction which also acts as a catalyst for the reaction. This means that as the amount of product increases, the reaction gets faster! Because Mn2+ autocatalyses the reaction, the rate increases as more catalyst is made. This gives an unusual concentration time graph. LO3: Explain with the aid of equations, how Fe2+ ions catalyse the reaction between I- and S2O82-, and how Mn2+ ions autocatalyse the reaction between C2O42- and MnO4-.