Understanding Covalent Bonding and Lewis Structures
Explore the concepts of covalent bonding, types of covalent substances, Lewis structures, and the importance of Lewis diagrams. Learn how to write Lewis structures with a step-by-step procedure and understand the significance of multiple bonds in molecules. Dive into the world of molecular formulas, structural formulas, and more through informative images and detailed explanations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
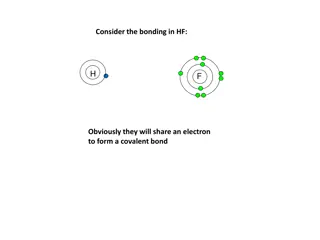
Electrons are shared! Occurs between nonmetal atoms with similar electronegativities Localized Electron model: A molecule is a group of atoms bound together by sharing pairs of electrons using the atomic orbitals of the free atoms. Bonding pairs in-between atoms Nonbonding pairs localized on one specific atom
Types of Formulas Molecular formulas: show which elements & how many atoms H2O, C6H12O6 Structural Formulas: above plus show connectivity. Show how the atoms are arranged and the type of bonding (use -, =, or ) Lewis diagrams: above plus show all the nonbonding valence electrons
Types of Covalent Substances Nonpolar covalent (no separation of charge) Polar covalent (separation of charge resulting from large differences in electronegativity) Network covalent: giant molecule or lattice Coordinate covalent: common in polyatomic ions with H
Lewis Structures Diagrams showing all the valence electrons of the molecule or polyatomic ion. Shows how the atoms are connected, the types of bonds, and the lone pairs.
Why Lewis structures are important!
Procedure for Writing Lewis Structures 1. 2. Count up the number of valence electrons & jot # down. Draw skeleton (gets easier with experience, use bonding capacities as guide lines) Compare, in the skeleton, the total number of electrons still needed for each atom to have an octet / duet to the # of electrons remaining for distribution after drawing skeleton. a) If deficient by 2, add one bond to skeleton, deficient by 4, add two bonds to skeleton, deficient by 6, add three bonds to skeleton (exceptions: Be, B, odd- electron molecules) b) If excess electrons, they may be placed on the CENTRAL atom if the the central atom is from row 3 or below in the periodic table (has d orbitals) 4. When the numbers match, distribute electrons from outside in. 5. Perform the two validity checks 3.
Multiple Bonds When drawing the Lewis structure of a molecule or ion, if every atom can t get an octet using single bonds, then invoke multiple bonding.
Resonance Occurs when more than one valid Lewis structure can be written for a species Atoms arranged the same way Electrons distributed differently A fix to the LE model
Molecular Shape & VSEPR Use the Lewis structure to determine the shape of many small molecules. Count up the electron domains on the central atom and the number of atoms bonded to the central atom Compare these two numbers (each can range from 2 to 6) to get molecular geometry.
What are electron domains in the Lewis Structure? An electron domain in the Lewis structure is any region of electron density. Each of the following counts as ONE electron domain. Single bond Double bond Triple bond Lone Pair Single electron on an atom in an odd-electron species.
Table for Molecular Geometry # of electron domains on central atom # of atoms bonded to central atom Shape Examples 2 2 Linear CO2, CS2, BeF2 3 3 Trigonal PLANAR BH3, BF3 3 2 Bent, bond angle = 120 SO2, O3 4 4 Tetrahedral, bond angles = 109 CH4, CF4, CH2F2 4 3 Trigonal Pyramid, bond angles = 107 NH3, NF3, PH3 4 2 Bent, bond angles = 105 H2O, H2S, H2Se, OF2
Table for Molecular Geometry # of electron domains on central atom # of atoms bonded to central atom Shape Examples 5 5 Trigonal BIPYRAMID PF5, AsF5 TeF4 ICl3 KrF2 SF6, SCl6, SeF6 IF5 KrF4 5 4 See-Saw 5 3 T-shape 5 2 Linear 6 6 Octahedral 6 5 Square Pyramid 6 4 Square Planar
Diatomic Molecules Always linear: 2 points make a line! For molecules with 3 or more atoms, use Lewis structure.
Page 16 4 domains on central O 2 atoms bonded to central O (4, 2) is bent with a 105 bond angle < ---- > < ---- > Note that in all 3 resonance structures for CO2, the central C atom has 2 electron domains & 2 atoms bonded to it. (2, 2) is linear. Bond angle = 180
Page 16 Central N atom has 4 electron domains & 3 atoms bonded to it. (4, 3) is trigonal pyramid with a 107 bond angle. Central C atom has 4 electron domains and 4 atoms bonded to it. (4, 4) is tetrahedral with a 109 bond angle.
Page 16 Central S atom has 3 electron domains and 3 bonded atoms. (3, 3) is trigonal planar.
Page 16 Starting to get some larger molecules. So think in terms of local geometry rather than global geometry. Around the C: (4, 4) which is tetrahedral. Around the O: (4, 2) which is bent.
Page 16 Central S atom has 4 electron domains and 2 atoms bonded to it. (4, 2) is Bent with a 105 bond angle. Hint: recognize that S is in the same column of the periodic table as O, so this molecule should have the same shape as H2O.
Page 16 The central atom is C with 4 electron domains & 4 atoms bonded to it. (4, 4) is tetrahedral with a 109 bond angle.
Using Lewis Structure to find Molecular shape What is the procedure? What do we mean by electron domains?
What factors influence bond energy? Type of bond: single vs. double vs. triple. Bond Length Size of the atoms involved in the bond. Polarity of the bond.
Comparison of Bond Strengths for Nonpolar Bonds Type of Bond Bond Length (pm) Avg. Bond Energy (kJ/mol) C-C C=C C C N-N N=N N N 154 134 120 145 125 110 346 612 835 163 418 945 Bond Strength: triple > double > single Note: the higher the bond energy, the shorter the bond.
Comparison of Bond Strengths for Nonpolar Bonds As bond order increases, there is more electron density between the two nuclei, increasing the attractive forces between the electrons & nuclei. ( A deeper potential well.) A triple bond is shorter & stronger than a single bond!
Bond length increases as atomic size increases Type of Bond Bond Length (pm) 92 127 142 161 Avg. Bond Energy (kJ/mol) 565 427 363 295 H-F H-Cl H-Br H-I As atomic radii increase, nuclei are farther away from each other. Attractive forces between electrons and nuclei are weakened by larger distances. Bond energy decreases.
Comparison of Bond Strengths as the bond becomes more polar EN Bond Length (pm) C-C 0 C-N 0.4 C-H 0.5 C-Cl 0.6 C-O 0.9 C-F 1.4 Type of Bond Avg. Bond Energy (kJ/mol) 346 305 413 339 358 485 154 147 110 176 143 141 C, N, O, F all in period 2; H in period 1; Cl in period 3
Bond Strength vs. Bond Polarity Given that bond lengths increase with increasing atomic size, there does seem to be some correlation between the polarity of a bond and the bond strength when the elements are in the same row. As polarity & % ionic character increase, bond strength increases.
Other Bond Dissociation Energies Ionic & metallic > covalent Ionic compounds are all solids at room temp. All metals but Hg are solids at room temp. but some have low melting points.
Coordinate Covalent Bonds One atom contributes both electrons to the bond. NH3 + H+ NH4+ H2O + H+ H3O+ The H+ has no electrons at all!
Properties Know the properties of the molecular covalent substances (the vast majority) Know the properties of the network covalent substances (C as diamond or graphite), SiO2, SiC.