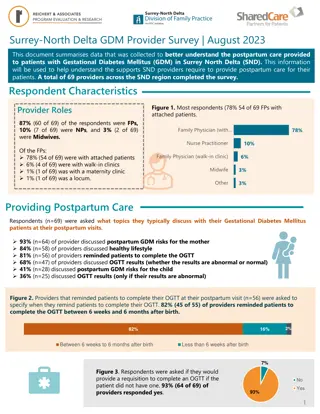
Understanding Gestational Diabetes: Evaluation and Diagnosis Guidance
Learn about the evaluation and diagnosis of gestational diabetes mellitus (GDM) in pregnant women, including testing criteria for undiagnosed diabetes and pregestational diabetes mellitus. Get insights into the definition of GDM, risk factors, diagnostic tests, and interpreting glucose levels to make accurate diagnoses.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
GESTATIONAL DIABETES MELLITUS Dr. Kashi Professor of Endocrinology Diabetes research center Mazandaran university of medical sciences
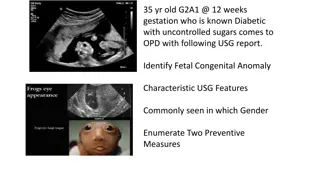
CASE 25 years old woman 6 weeks pregnant W=90 H=150 History of PCO What is your advice for GDM evaluation in this woman?
DEFINITION For many years, GDM was defined as any degree of glucose intolerance that was first recognized during pregnancy This definition facilitated a uniform strategy for detection and classification of GDM, but it was limited by imprecision. The ongoing epidemic of obesity and diabetes has led to more type 2 diabetes in women of childbearing age, with an increase in the number of pregnant women with undiagnosed type 2 diabetes . Because of the number of pregnant women with undiagnosed type 2 diabetes, it is reasonable to test women with risk factors for type 2 diabetes
Test for undiagnosed diabetes at the first prenatal visit in those with risk factors, using standard diagnostic criteria
Testing should be considered in overweight or obese (BMI 25 kg/m2) adults who have one or more of the following risk factors: A1C 5.7% (39 mmol/mol), IGT, or IFG on previous testing first-degree relative with diabetes women who were diagnosed with GDM history of CVD hypertension ( 140/90 mmHg or on therapy for hypertension) HDL cholesterol level ,35 mg/dL and/or a triglyceride level .250 mg/dL women with polycystic ovary syndrome physical inactivity other clinical conditions associated with insulin resistance (e.g., severe obesity, acanthosis nigricans)
Pregestational diabetes mellitus FPG 126 mg/dL (7.0 mmol/L). Fasting is defined as no caloric intake for at least 8 h.* OR 2-h PG 200 mg/dL during an 75 gr OGTT. * OR A1C 6.5% OR In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose 200 mg/dL . *In the absence of unequivocal hyperglycemia, results should be confirmed by repeat testing.
Case(cont.) FBS=93 mg/dl HBA1C=5.6% 2H75PG=135 mg/dl What is the diagnosis?
Gestational DM diabetes that is first diagnosed in the second or third trimester of pregnancy that is not clearly either preexisting type 1 or type 2 diabetes
Test for gestational diabetes mellitus at 24 28 weeks of gestation in pregnant women not previously known to have diabetes
DIAGNOSIS GDM diagnosis can be accomplished with either of two strategies: 1. One-step 75-g OGTT 2. Two-step approach with a 50-g (nonfasting) screen followed by a 100-g OGTT for those who screen positive
One-step strategy Perform a 75-g OGTT, with plasma glucose measurement when patient is fasting and at 1 and 2 h, at 24-28 weeks of gestation in women not previously diagnosed with overt diabetes. The OGTT should be performed in the morning after an overnight fast of at least 8 h. The diagnosis of GDM is made when any of the following plasma glucose values are met or exceeded: Fasting: 92 mg/dL (5.1 mmol/L) 1 h: 180 mg/dL (10.0 mmol/L) 2 h: 153 mg/dL (8.5 mmol/L)
Two-step strategy Step 1: Perform a 50-g GLT (nonfasting), with plasma glucose measurement at 1 h, at 24 28 weeks of gestation in women not previously diagnosed with overt diabetes. If the plasma glucose level measured 1 h after the load is 130 mg/dL, proceed to a 100-g OGTT. Step 2: The 100-g OGTT should be performed when the patient is fasting.
Carpenter/Coustan criteria The diagnosis of GDM is made if at least two of the following four plasma glucose levels (measured fasting and 1 h, 2 h, 3 h after the OGTT) are met or exceeded: Fasting 95 mg/dL 1 h 180 mg/dL 2 h 155 mg/dL 3 h 140 mg/Dl Two step vs One step strategy prevalences (6% vs. 15% to 20%) (4.2% vs. 38.5%) in Mazandaran
GDM Complications In contrast to women with pregestational diabetes, women with true GDM typically do not have diabetes-related vasculopathy or an increased risk of infants with congenital malformations because of the short duration of the disorder and late pregnancy onset.
GDM Complications Short term: Large for gestational age (LGA) infant andmacrosomia Preeclampsia Polyhydramnios Stillbirth Neonatal morbidity(hypoglycemia, hyperbilirubinemia, hypocalcemia, hypomagnesemia, polycythemia, respiratory distress, and/or cardiomyopathy)
GDM Complications Long-term : Offspring obesity, impaired glucose tolerance, or metabolic syndrome maternal development of type 2 diabetes, including diabetes-related vascular disease
Case (cont.) One step GTT results: FBS=90 mg/dl BS1HP 75 gr G=190mg/dl BS2HP75gr G=150mg/dl What is the diagnosis? What is the next step?
When initially diagnosed with GDM, patients are asked to measure their blood glucose concentration at least four times daily (fasting and one or two hours after the first bite of each meal)
Lifestyle Management After diagnosis, treatment starts with medical nutrition therapy, physical activity, and weight management depending on pregestational weight 70 85% of women diagnosed with GDM under two step strategy can control GDM with lifestyle modification alone; it is anticipated that this proportion will be even higher if the lower one step diagnostic thresholds are used.
GLYCEMIC TARGETS IN PREGNANCY the ADA-recommended targets for women with type 1 or type 2 diabetes (the same as for GDM; described below) are as follows: Fasting 95 mg/dL and either One-hour after starting the meal 140 mg/dL or Two-hour after starting the meal 120 mg/dL
Some experts have suggested medical nutritional therapy for women who do not meet oral GTT criteria for GDM, but have fasting blood glucose concentrations >90 mg/dL , an abnormal glucose challenge test, or one abnormal value on the 100 g, three-hour oral GTT
Case (cont.) FBS=93mg/dl BS1H after breakfast=150mg/dl BS1H after breakfast=120mg/dl BS1H after breakfast=140mg/dl What is the best intervention?
Pharmacologic Therapy Insulin is the first-line agent recommended for treatment of GDM While individual randomized controlled trials support the efficacy and short-term safety of metformin and glyburide for the treatment of GDM, both agents cross the placenta. Long-term safety data are not available for any oral agent. Patients treated with oral agents should be informed that they cross the placenta, and although no adverse effects on the fetus have been demonstrated, long-term studies are lacking.
Sulfonylureas Concentrations of glyburide in umbilical cord plasma are approximately 70% of maternal levels . Glyburide may be associated with a higher rate of neonatal hypoglycemia and macrosomia than insulin or metformin .
Metformin Metformin may be associated with a lower risk of neonatal hypoglycemia and less maternal weight gain than insulin; however, metformin may slightly increase the risk of prematurity. Umbilical cord blood levels of metformin are higher than simultaneous maternal levels. None of these studies or meta-analyses evaluated long-term outcomes in the offspring. Nearly half of patients with GDM who were initially treated with metformin in a randomized trial needed insulin in order to achieve acceptable glucose control.
INSULIN Insulin should be initiated (or increase the dose) when one-third of fasting or postprandial glucose levels exceed the target in a given week. It is reasonable for women with fetuses with large abdominal circumferences to receive insulin to decrease the risk of macrosomia, even if they have no or mild hyperglycemia It is reasonable to relax self-glucose monitoring and initiation of insulin for mild hyperglycemia in women whose fetuses have a small abdominal circumference (<75th percentile). Withholding insulin when there is no evidence of increased somatic growth may limit the risk of iatrogenic growth restriction
Initiating Insulin Therapies with Mild Hyperglycemia Glycemic Derangement Persistent FPG >95 mg/dL <120 mg/dL Suggested Insulin Type and Dose Start 8 - 20 units NPH at bedtime (0.165 or 0.2 units per kg. actual body weight) Start 2 - 4 units lispro or aspart pre- breakfast One hour post breakfast plasma value >135 mg/dL <180 mg/dL One hour post lunch plasma value >135 mg/dL <180 mg/dL Add 6-10 units NPH to pre-breakfast injection (And eat lunch 4-5 hrs after breakfast) OR Give 2- 4 units lispro or aspart pre-lunch Give 2- 4 units lispro or aspart pre-lunch One hour post dinner plasma value >135mg/dL <180mg/dL
Determining Effective Insulin Regimens Glycemic Derangement If pre-dinner plasma values are >100 mg/dL Suggested Insulin Type and Dose Increase AM dose of NPH if dinner is within 8 hrs. of the AM injection. If the patient eats a late Dinner, NPH with lunch is often more effective - add 4 units NPH at lunch and titrate as needed Titrate these doses up or down by 1 - 4 units based on BG values that are out of range for 2 to 3 days at a time.
Suggested Starting Total Daily Insulin During Pregnancy 50 percent as NPH insulin (given in three equal doses before breakfast, before dinner and before bedtime) and 50 percent as three preprandial rapid-acting insulin injections.
Timing and route of delivery For women with gestational diabetes mellitus (GDM) requiring pharmacologic therapy, it is suggested a standard form of antenatal fetal testing ,twice weekly, using a nonstress test with an amniotic fluid index, starting at 32 weeks of gestation induction of labor is suggested at 39 weeks of gestation . Potential benefits include lower rates of macrosomia and large for gestational age (LGA) infants, lower rates of shoulder dystocia, and lower rates of cesarean delivery.
single third trimester ultrasound is needed to screen for macrosomia. Scheduled cesarean delivery to avoid birth trauma is typically offered to women with GDM and estimated fetal weight 4500 grams. Such women should be counseled about the poor predictive ability of ultrasound estimates of fetal weight and the risks and benefits of cesarean delivery in the current and future pregnancies
PRGNANCY AND DRUG CONSIDERATIONS In normal pregnancy, blood pressure is lower than in the nonpregnant state. In a pregnancy complicated by diabetes and chronic hypertension, target goals for systolic blood pressure 120 160 mmHg and diastolic blood pressure 80 105 mmHg are reasonable .Lower blood pressure levels may be associated with impaired fetal growth.
During pregnancy, treatment with ACE inhibitors and angiotensin receptor blockers is contraindicated because they may cause fetal renal dysplasia, oligohydramnios, and intrauterine growth restriction. Antihypertensive drugs known to be effective and safe in pregnancy include methyldopa, labetalol, diltiazem, clonidine, and prazosin. Chronic diuretic use during pregnancy is not recommended as it has been associated with restricted maternal plasma volume, which may reduce uteroplacental perfusion . On the basis of available evidence, statins should also be avoided in pregnancy .