Understanding Health-Related Quality of Life in Drug-Resistant TB Patients
Explore the impact of adverse events on the health-related quality of life in drug-resistant tuberculosis (DR-TB) patients in Johannesburg, South Africa. This study aims to measure HRQoL using the SF-36 Health Survey, describe adverse events experienced during treatment, and compare the HRQoL between patients reporting adverse events and those who do not. The research is conducted by the Health Economics and Epidemiology Research Office at Wits Health Consortium, University of the Witwatersrand.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Poorer Health Poorer Health- -Related Quality Related Quality of Who Report Adverse Events Who Report Adverse Events while on Drug Drug- -Resistant Resistant TB in Johannesburg, South Africa TB in Johannesburg, South Africa of Life Among Patients Life Among Patients while on Treatment Treatment for for Denise Evans, Tembeka Sineke Tembeka Sineke, Kate Schnippel, Helena van Aswegen, Rebecca Berhanu, Lawrence Long, Elisabeth L nnermark, Sydney Rosen PHASA Conference 22-29 September 2016 PHASA Confere Health Economics and Epidemiology Research Office HE RO 2 Wits Health Consortium University of the Witwatersrand nce 22-29 September 2016
Background South Africa bears a disproportionate share of the world s epidemic of drug-resistant tuberculosis (DR-TB). Adverse events are common during treatment of DR-TB. Some harm quality of life (e.g. abdominal pain, nausea) Some are disabling (e.g. irreversible hearing loss) Some are life threatening (e.g. renal failure, psychosis, seizures). Little is known about health-related quality of life (HRQoL) of patients receiving treatment for DR-TB or the effect of adverse events on HRQoL. Health Economics and Epidemiology Research Office HE RO 2 Wits Health Consortium University of the Witwatersrand
Aims Measure the HRQoL of DR-TB patients receiving outpatient treatment and care using a standardized tool (Short Form 36 Health Survey). Describe the experience of adverse events (AEs) by patients receiving DR-TB treatment including frequency and characteristics of AEs using a standardized tool. Compare health-related quality of life among DR-TB patients who reported an adverse event versus those who do not report an adverse event in the past 4 weeks.
Methods Cross-sectional survey of a sub-sample of patients enrolled in a prospective cohort study. Cohort Cohort study study enrolled enrolled adult adult ( ( 18 18) ) patients patients who who: : - Documented laboratory diagnosis of RIF resistant TB. - Initiated DR-TB treatment at the specialized, outpatient TB Focal Point (FP) of Helen Joseph Hospital, Johannesburg, South Africa after March 2013. - Provide informed consent for medical file review. Sample Sample for for HRQoL HRQoL survey survey: : Conveniently sampled patients enrolled in cohort study after February 2015. Willing to participate and provide informed consent. Outcome Outcome: : Poor HRQoL defined as a low Mental Component Score (MCS) or Physical Component Score (PCS): according to scoring instructions and using the median as a cut-off point. Health Economics and Epidemiology Research Office HE RO 2 Wits Health Consortium University of the Witwatersrand
Methods (Continued) Survey instruments Survey instruments - SF SF- -36 weeks. 36, questionnaire asking about health-related quality of life in the past 4 - Part A Part A: All patients, recent symptoms in past four weeks. - Part B Part B: Detailed questions about specific AEs reported in Part A (e.g. hearing loss, psychosis or depression, vertigo, and/or (iv) peripheral neuropathy) - Part Part C C: Validated hearing loss screening tool (HHIE-S) for patients who reported hearing loss in Part A. Statistical analysis Statistical analysis - Log-binomial regression was used to test the association between reporting an adverse event in the last four weeks and a low MCS or PCS. Models adjusted for age, gender, and time on DR-TB treatment. - Scales were compared to SF-36 scales for healthy adults in Johannesburg from a published study (van Aswegen et al, 2011). Health Economics and Epidemiology Research Office HE RO 2 Wits Health Consortium University of the Witwatersrand
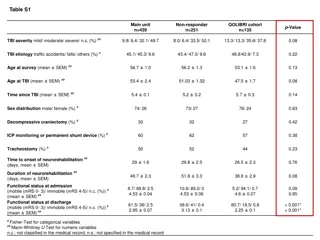
Characteristics of Patients with Drug-Resistant Tuberculosis Characteristic Gender Description Male Female (N=57) 33 (57.9%) 24 (42.1%) 37 (29-40) 26 (45.6%) 31 (54.6%) 47 (82.5%) 10 (17.5%) n, % Age, years Median, IQR n, % 35 >35 Primary/no education Higher Education n, % Employment Employed Not Employed RR-TB MDR-TB n, % 35 (61.4%) 22 (38.6%) 42 (73.7%) 15 (26.3%) 15.6 (8.5 - 20.2) Classification n,% Time on TB treatment, months Median, IQR HIV status Positive Negative n, % 49 (86.0%) 8 (14.0%) 78 (29 - 233) 21/49 (42.9%) 28/49 (57.1%) 20.6 (10.5 - 29.8) Baseline CD4 (n=35) On ART at start of RR-TB treatment Median, IQR n, % Yes No Time on ART treatment, months Median, IQR
Adverse Events, Stratified by Age Category Adverse Events More than a third of patients (21/57, 37%) reported an adverse event in the past four weeks. Total of 24 adverse events described: 7 hearing loss, 2 psychosis or depression, 5 vertigo, and 10 peripheral neuropathy. 50% 45% 40% 35% 30% 35 years 25% > 35 years 20% 15% 10% 5% 0% Peripheral neuropathy Hearing loss Vertigo Depression
SF-36 Scales and Mental and Physical Health Component Summary Scores SF-36 0-100 scales Adverse event not reported (n=36) Adverse event reported (n=21) P value Mean SD Alpha* Mean SD Alpha* Physical functioning 94.7 11.7 0.868 72.1 30.3 0.872 0.0004 Role physical 82.6 29.8 0.839 45.2 45.8 0.837 0.001 Role emotional 80.6 35.9 0.836 30.5 45.8 0.854 0.001 Energy/vitality 64.7 18.1 0.835 50.5 21.8 0.855 0.009 Emotional well-being 72.6 16.6 0.818 58.1 16.8 0.843 0.002 Social functioning 80.2 25.4 0.830 60.7 23.1 0.849 0.002 Pain 84.4 23.5 0.820 69.1 26.7 0.858 0.030 General health 75.7 13.1 0.844 68.8 12.5 0.882 0.041 Norm-based scale Mental health component summary (MCS) Physical health component summary (PCS) 46.2 11.4 n/a 33.3 12.3 n/a 0.001 55.4 5.8 n/a 48.4 9.2 n/a 0.007
Summary Scores Patients who reported an adverse event (n=21) had significantly lower scales for physical functioning, emotional role, energy, and social functioning and were more likely to have a lower MCS and PCS. Proportion with lower MCS Crude RR and 95% CI Adjusted RR and 95% CI No adverse event 12/36 1.0 (reference) 1.0 (reference) Adverse event 16/21 3.3 (1.40 7.82) 3.7 (1.62 8.62) Proportion with lower PCS Crude RR and 95% CI Adjusted RR and 95% CI No adverse event 14/36 1.0 (reference) 1.0 (reference) Adverse event 15/21 2.4 (1.09 5.33) 2.6 (1.19 5.69) Health Economics and Epidemiology Research Office HE RO 2 Wits Health Consortium University of the Witwatersrand
SF-36 Scales for Patients on DR-TB Treatment and Healthy Adults 100 80 SF-36 scales (0 - 100) 60 40 20 0 Adverse event not reported Healthy adults Adverse event reported
Conclusions Patients who reported an adverse event during DR-TB treatment reported poorer HRQoL (MCS and PCS) than those who did not. Based on the SF-36 scales, patients with adverse events reported much poorer HRQoL than healthy adults. These findings are likely to have implications for patients clinical, social and economic wellbeing. Study had several limitations: Small sample size continuing to enroll patients until December 2016. Did not have a comparison group that was representative of study population. Study population has high rates of HIV co-infection (86%), with more complicated HIV/ART cases. Health Economics and Epidemiology Research Office HE RO 2 Wits Health Consortium University of the Witwatersrand
Next steps SF-36 could be used as a screening tool to identify patients who require referral (e.g. depression or hearing loss) or targeted interventions. We plan to assess whether patients with poor HRQoL, in particular those with poor MCS or poor PCS, have poorer treatment outcomes (e.g. treatment failure, LTFU) and identify which patients may require rehabilitation (e.g. hearing loss) after treatment. We plan to enroll comparison cohorts.
Acknowledgments Denise Evans Sydney Rosen Lawrence Long Tembeka Sineke Kathryn Schnippel Heleen van Aswegen Rebecca Berhanu Elisabet L nnmark Patients and staff at CHRU and Themba Lethu Clinic Helen Joseph Hospital Health Economics and Epidemiology Research Office HE RO 2 Wits Health Consortium University of the Witwatersrand This presentation was made possible by the generous support of the American people through Cooperative Agreement AID 674-A-12-00029 from the United States Agency for International Development (USAID). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government