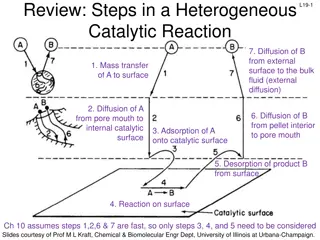
Understanding Internal and External Diffusion in Chemical Reactions
Explore the dynamics of internal and external diffusion impacting reactant concentration in chemical reactions. Learn how to derive new rate equations considering mass transfer and reaction rates. Gain insights into molar balance at catalyst surfaces and overall reaction rates accounting for diffusion resistance.
Uploaded on | 1 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
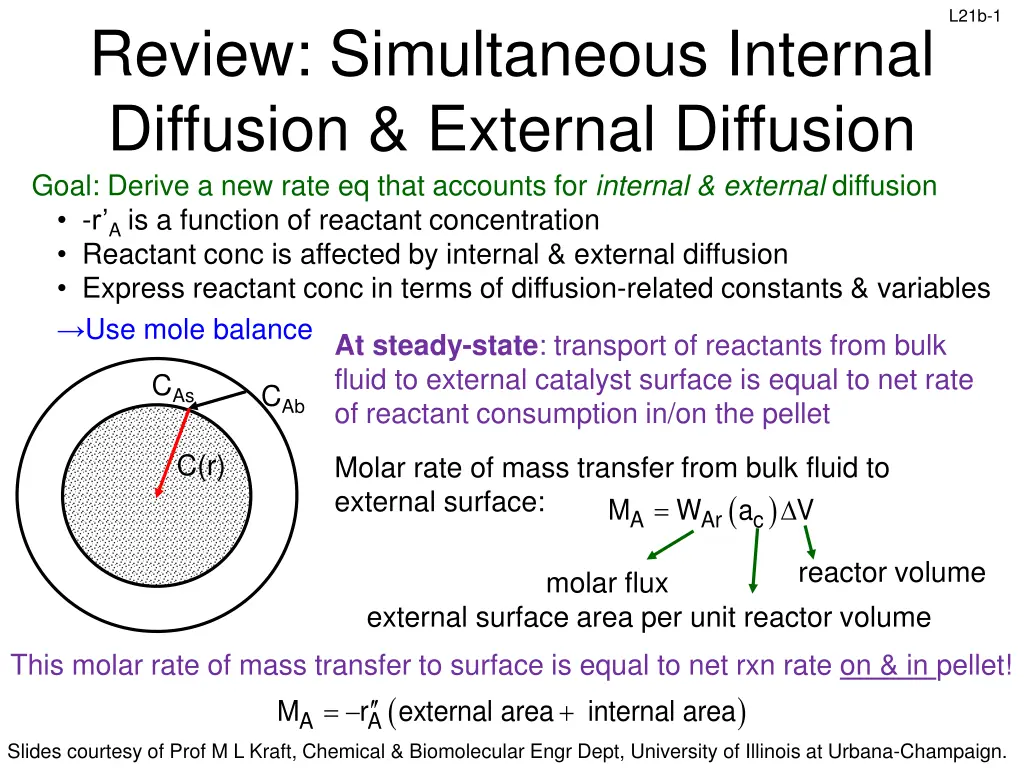
L21b-1 Review: Simultaneous Internal Diffusion & External Diffusion Goal: Derive a new rate eq that accounts for internal & external diffusion -r A is a function of reactant concentration Reactant conc is affected by internal & external diffusion Express reactant conc in terms of diffusion-related constants & variables Use mole balance At steady-state: transport of reactants from bulk fluid to external catalyst surface is equal to net rate of reactant consumption in/on the pellet CAs CAb C(r) Molar rate of mass transfer from bulk fluid to external surface: M ( ) = W a V A Ar c reactor volume molar flux external surface area per unit reactor volume This molar rate of mass transfer to surface is equal to net rxn rate on & in pellet! ( A A M r external area = ) + internal area Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Review: Basic Molar Balance at Spherical Pellet Surface Flux: bulk to external surface S.A. ( A Ar c A r R = L21b-2 Actual rxn rate per unit total External S.A. external + internal S.A. x = x ) = = + M W a V r a V a b S V c ac: external surface area per reactor volume (m2/m3) V: reactor volume (m3) : porosity of bed (void fraction) -r A: rate of reaction per unit surface area (mol/m2 s) -r A: mol/g cat s Sa: surface area of catalyst per unit mass of catalyst (m2/g cat) b: bulk density, catalyst mass/ reactor volume b= c(1- ) r' r'' S r r' S k' k' '' k = = per mass cat -rA: mol/volume s per = = = = a c r r'' S k'' volume A A a A A c A A S per surface k k area n n a n n c n n a c Cancel out V &ac 0 since external surface area usually <<< internal surface area (surface area of internal pores) M W = = A a b r S = a A Ar c r R Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-3 Review: Overall Molar Rate of Reaction M W = For external mass transport: = = W a r S A Arr c A a b R ( ) = k C C Ar c Ab As r R = Internal diffusion resistance is significant, so the reactant conc at the internal surface is lower that the reactant conc at the external surface: : internal effectiveness factor r ' ' observed rxn rate rxn rate if no internal dif For a 1st order rxn: -r A=- k1CAs ( ) A = = = r'' r'' As A r'' f l m t i i As Plug flux & 1st order rxn rate back into the mass balance, solve for CAs: k C a + ( ) c Ab c = = 1 As k C = M k C C a a b S C A c Ab As c As k'' S c c k a 1 a b Insert CAs into r A= k1CAs: 1 c c k k a C k a + Overall 1st order rxn rate with internal & external diffusion Ab = r A 1 a b k S c c Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-4 Review: Overall Effectiveness Factors Remember, the internal effectiveness factor isbased on CAs actualoverallrate of reaction = rate of rxnif entire interior surface were exposed to the external surface conditions The overall effectiveness factor is based on CAb: Omega actualoverallrate of reaction = rate of reactionif entire interior surface were ex p os ed to th e bul k co ndit io s n 1 Ab k C k S k C r + 1 k c c a A = 1 a b = = r + 1 1 a b k S c c k a Ab 1 Ab ( ) = Ab r r'' Put into design eq to account for internal & external diffusion A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-5 Review: Observed Rxn Rate Variation vs FT0, dp & T ( ) A c Ab As r' k C C k = diffusion limited: 1 2 13 External U d D p AB d = + 2 0.6 c D p AB c 1 a k S D c 1 a k S D 3 Internal diffusion = = r k C As a S R coth R 1 A r c 1 a k D S limited: 2 e e R e Surface reaction -r A=kCA Variation of Reaction Rate with: Superficial velocity Type of Limitation Particle size Temperature U1/2 dp-3/2 Linear External Independent dp-1 Exponential Internal Independent Independent Exponential Surface reaction Whether the rate varies when FT0 or particle size changes indicates tells us whether external diffusion, internal diffusion, or the surface rxn is limiting (slowing down) the Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign. observed rate
Review: Rxn Rate Variation vs Reactor Conditions When the observed rate of a reaction is limited by external diffusion, internal diffusion, or the surface rxn, the observed reaction kinetics are: L21b-6 1 2 13 Rate for external diff limited rxn: U d D p ( ) AB d = = + r' k C C k 2 0.6 A c Ab As c D p AB kc: mass transfer coefficient DAB: diffusivity (m2/s) U: free-stream velocity (m/s), to flow rate (FT, FT0) for constant CA0 : kinematic viscosity (m2/s); = dp: diameter : fluid density (kg/m3) : viscosity c 1 a k S D c 1 a k S D 3 Rate for internal diff limited rxn: = = r k C As a S R coth R 1 A r c 1 a k D S 2 e e R e R: radius at particle surface De: effective diffusivity ( ) actual observed overallrate of rxn rate of rxnif entire interior surface were exposed to C = & T As s Rate for surface reaction limited rxn: -r A = kCA Whether the rate varies when FT0 (at constant CT0) or particle size changes indicates tells us whether external diffusion, internal diffusion, Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign. or the surface rxn is limiting (slowing down) the observed rate
L21b-7 Observed Rxn Rate vs FT0, dp & T 1 2 13 Rate for external diff limited rxn: U d D p ( ) AB d = = + r' k C C k 2 0.6 A c Ab As c D p AB U: free-stream velocity (m/s), to flow rate (FT, FT0) for constant CA0 3 k S D R: radius at particle surface dp: diameter c 1 a k S D c 1 a k S D Rate for internal diff limited rxn: = = r k C As a S R coth R 1 A r 2 c 1 a e e R e Rate for surface reaction limited rxn: -r A= kCA According to these equations, if we increase the flow rate (FT0) without increasing the concentration of reactants in the feed, the observed rxn rate will increase if the rxn is limited (slowed down) by: a. External diffusion b. Internal diffusion c. The surface reaction d. Either external & internal diffusion e. Any of these (external diffusion, internal diffusion, or surface reaction) Free-stream velocity (U), which is to flow rate for constant CA0, is only in rate eq for a external diffusion limited reaction Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-8 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 -rA (mol/g cat*h) 10 8 6 4 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by external diffusion? External diffusion limits the observed rate when increasing FT0 increases r A Need to find the points that have the same T and dp. If the rate increases when Fto increases, the trial at the LOWER flow rate is limited by external diffusion Trial with T = 400K, dp= 0.8 cm & FT0= 1000 mol/h has a lower rate than the trial with T = 400K, dp= 0.8 cm & FT0= 1500 mol/h Thus, rate is limited by external diffusion when T = 400K, dp= 0.8 cm & FT0= 1000 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-9 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 -rA (mol/g cat*h) 10 8 6 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by external diffusion? External diffusion limits the observed rate when increasing FT0 increases r A Need to find the points that have the same T and dp. If the rate increases when Fto increases, the trial at the LOWER flow rate is limited by external diffusion Trial with T = 400K, dp= 0.8 cm & FT0= 1500 mol/h has a lower rate than the trial with T = 400K, dp= 0.8 cm & FT0= 2000 mol/h Thus, rate is limited by external diffusion when T = 400K, dp= 0.8 cm & FT0= 1500 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-10 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 -rA (mol/g cat*h) 10 8 6 ext diff lim ext diff lim 4 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by external diffusion? External diffusion limits the observed rate when increasing FT0 increases r A Need to find the points that have the same T and dp. If the rate increases when Fto increases, the trial at the LOWER flow rate is limited by external diffusion Trial with T = 400K, dp= 0.8 cm & FT0= 2000 mol/h has a lower rate than the trial with T = 400K, dp= 0.8 cm & FT0= 3500 mol/h Thus, rate is limited by external diffusion when T = 400K, dp= 0.8 cm & FT0= 2000 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-11 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 -rA (mol/g cat*h) 10 ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by external diffusion? External diffusion limits the observed rate when increasing FT0 increases r A Need to find the points that have the same T and dp. If the rate increases when Fto increases, the trial at the LOWER flow rate is limited by external diffusion Trial with T = 400K, dp= 0.8 cm & FT0= 3500 mol/h has the same rate as the trial with T = 400K, dp= 0.8 cm & FT0= 4000 mol/h Rate is NOT limited by external diffusion when T = 400K, dp= 0.8 cm & FT0= 3500 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign. or T = 400K, dp= 0.8 cm & FT0= 4000 mol/h
L21b-12 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 -rA (mol/g cat*h) 10 ext diff lim 8 6 ext diff lim ext diff lim 4 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by external diffusion? External diffusion limits the observed rate when increasing FT0 increases r A Need to find the points that have the same T and dp. If the rate increases when Fto increases, the trial at the LOWER flow rate is limited by external diffusion For all remaining trials, increasing FT0 does not increase the reaction rate, so no other trial conditions are external diffusion limited. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-13 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 -rA (mol/g cat*h) 10 ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by internal diffusion? Internal diffusion limits the observed rate when decreasing dp increases r A Need to find the points that have the same T. If the rate increases when dp decreases but does not change with FT0, the trial at the largerdp is limited by internal diffusion Trial with T = 400K, dp= 0.8 cm & FT0= 3500 mol/h has a lower rate than the trial with T = 400K, dp= 0.6 cm & FT0= 3500 mol/h Thus, rate is limited by internal diffusion when T = 400K, dp= 0.8 cm & FT0= 3500 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-14 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by internal diffusion? Internal diffusion limits the observed rate when decreasing dp increases r A Need to find the points that have the same T. If the rate increases when dp decreases but does not change with FT0, the trial at the largerdp is limited by internal diffusion Trial with T = 400K, dp= 0.8 cm & FT0= 4000 mol/h has a lower rate than the trial with T = 400K, dp= 0.6 cm & FT0= 4000 mol/h Thus, rate is limited by internal diffusion when T = 400K, dp= 0.8 cm & FT0= 4000 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-15 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 int diff lim -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by internal diffusion? Internal diffusion limits the observed rate when decreasing dp increases r A Need to find the points that have the same T. If the rate increases when dp decreases but does not change with FT0, the trial at the largerdp is limited by internal diffusion Trial with T = 400K, dp= 0.6 cm & FT0= 3500 mol/h has a lower rate than the trial with T = 400K, dp= 0.2 cm & FT0= 3500 mol/h Thus, rate is limited by internal diffusion when T = 400K, dp= 0.6 cm & FT0= 3500 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-16 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 int diff lim int diff lim -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by internal diffusion? Internal diffusion limits the observed rate when decreasing dp increases r A Need to find the points that have the same T. If the rate increases when dp decreases but does not change with FT0, the trial at the largerdp is limited by internal diffusion Trial with T = 400K, dp= 0.6 cm & FT0= 4000 mol/h has a lower rate than the trial with T = 400K, dp= 0.2 cm & FT0= 4000 mol/h Thus, rate is limited by internal diffusion when T = 400K, dp= 0.6 cm & FT0= 4000 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-17 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 int diff lim 12 int diff lim int diff lim -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by internal diffusion? Internal diffusion limits the observed rate when decreasing dp increases r A Need to find the points that have the same T. If the rate increases when dp decreases but does not change with FT0, the trial at the largerdp is limited by internal diffusion Trial with T = 400K, dp= 0.2 cm & FT0= 3500 mol/h has a lower rate than the trial with T = 400K, dp= 0.1 cm & FT0= 3500 mol/h Thus, rate is limited by internal diffusion when T = 400K, dp= 0.2 cm & FT0= 3500 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-18 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 int diff lim int diff lim 12 int diff lim int diff lim -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by internal diffusion? Internal diffusion limits the observed rate when decreasing dp increases r A Need to find the points that have the same T. If the rate increases when dp decreases but does not change with FT0, the trial at the largerdp is limited by internal diffusion Trial with T = 400K, dp= 0.2 cm & FT0= 4000 mol/h has a lower rate than the trial with T = 400K, dp= 0.1 cm & FT0= 4000 mol/h Thus, rate is limited by internal diffusion when T = 400K, dp= 0.2 cm & FT0= 4000 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-19 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 int diff lim int diff lim int diff lim FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 int diff lim int diff lim -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by internal diffusion? Internal diffusion limits the observed rate when decreasing dp increases r A Need to find the points that have the same T. If the rate increases when dp decreases but does not change with FT0, the trial at the largerdp is limited by internal diffusion Trial with T = 400K, dp= 0.1 cm & FT0= 3500 mol/h has the SAME rate as the trial with T = 400K, dp= 0.05 cm & FT0= 3500 mol/h Thus, rate is limited by internal diffusion when T = 400K, dp= 0.2 cm & FT0= 4000 mol/h Rate is NOT limited by internal diffusion when T = 400K, dp= 0.1 cm & FT0= 3500 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign. or T = 400K, dp= 0.05 cm & FT0= 3500 mol/h
L21b-20 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 int diff lim int diff lim int diff lim FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 int diff lim int diff lim -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by internal diffusion? Internal diffusion limits the observed rate when decreasing dp increases r A Need to find the points that have the same T. If the rate increases when dp decreases but does not change with FT0, the trial at the largerdp is limited by internal diffusion For all remaining trials, decreasing dp does not increase the reaction rate, so no other trial conditions are internal diffusion limited. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-21 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 int diff lim int diff lim int diff lim FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 int diff lim int diff lim -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by the surface reaction? The surface reaction limits the reaction rate when the observed rxn rate increases when we increase T, but it does not increase when we decrease dp or increase FT0 without increasing CT0 For all remaining trials, neither decreasing dp nor increasing FT0 increases the reaction rate. Therefore, the surface reaction limits (slows down) the rates of the remaining trial conditions. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-22 The graph below shows the reaction rates obtained when the irreversible, liquid- phase, catalytic reaction A B was carried out in a PBR using the indicated catalyst dp, T, and FT0. CA0 was the same in each trial. 16 int diff lim int diff lim int diff lim SRL FT0=4000 mol/h, T=400K FT0=4000 mol/h, T=300K FT0=3500 mol/h, T=400K FT0=3500 mol/h, T=300K FT0=1000 mol/h, T=400K FT0=1500 mol/h, T=400K FT0=2000 mol/h, T=400K 14 12 int diff lim int diff lim -rA (mol/g cat*h) 10 int diff lim ext diff lim 8 6 ext diff lim 4 ext diff lim 2 SRL 0 0.0 0.1 0.2 0.3 0.4 dp (cm) 0.5 0.6 0.7 0.8 0.9 Which, if any, of the conditions shown (flow rates, T, and dp) is the reaction limited by the surface reaction? For all remaining trials, neither decreasing dp nor increasing FT0 increases the reaction rate. Therefore, the surface reaction limits (slows down) the rates of the remaining trial conditions. Surface reaction limited (SRL): T = 400K, dp= 0.1 cm & FT0= 3500 mol/h, T = 400K, dp= 0.1 cm & FT0= 4000 mol/h, T = 400K, dp= 0.05 cm & FT0= 4000 mol/h, T = 300K, all dp tested, & FT0= 4000 mol/h & T = 300K, all dp tested, & FT0= 3500 mol/h T = 400K, dp= 0.05 cm & FT0= 3500 mol/h, Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-23 The catalytic reaction A B takes place in a fixed bed reactor containing spherical porous catalyst X22. The overall rxn rates at a point in the reactor are shown in the graph below. For which, if any, of the conditions shown (flow rates and temps) is the reaction limited by external diffusion? -r A (mol/gcat s) External diffusion limited where r A linearly when T Variation of Reaction Rate with: Superficial velocity Superficial velocity Variation of Reaction Rate with: Type of Limitation Limitation Type of Particle size Particle size Temperature Temperature U1/2 U1/2 dp-3/2 dp-1 dp-1 Linear Linear External External dp-3/2 Independent Independent Exponential Exponential Internal Internal Independent Independent Independent Independent Exponential Exponential Surface reaction Surface reaction For FT0 = 10 mol/h, the rate of rxn increases approximately linearly with T over the entire temperature range- external diffusion limited at FT0 = 10 and all T For FT0 = 100 mol/h, the rate of rxn increases ~linearly with T when T > 360K. The reaction is external diffusion limited when FT0 = 100 & T> 360K Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-24 The catalytic reaction A B takes place in a fixed bed reactor containing spherical porous catalyst X22. The overall rxn rates at a point in the reactor are shown in the graph below. For which, if any, of the conditions shown (flow rates and temps) is the reaction limited by surface reaction rate? Surface rxn limited when r Aincreases exponentially with T but independent of superficial velocity (flow!) Rxn rates exp with T but not FT0 for FT0 = 100, 1000 & 5000 mol/h at T< 360K -r A (mol/gcat s) ext diff lim Variation of Reaction Rate with: Superficial velocity Superficial velocity Variation of Reaction Rate with: Type of Limitation Limitation Type of Particle size Particle size Temperature Temperature U1/2 U1/2 dp-3/2 dp-1 dp-1 dp-3/2 Linear Linear External External Independent Independent Exponential Exponential Internal Internal Independent Independent Independent Independent Exponential Exponential Surface reaction Surface reaction For conditions FT0 = 100, 1000 & 5000 mol/h at T< 360K, rxn rate is independent of FT0 but exponentially dependent on T surface reaction limited Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-25 The catalytic reaction A B takes place in a fixed bed reactor containing spherical porous catalyst X22. The overall rxn rates at a point in the reactor are shown in the graph below. For which, if any, of the conditions shown (flow rates and temps) is the reaction limited by surface reaction rate? Surface rxn limited when r Aincreases exponentially with T but independent of velocity For FT0 = 1000 & 5000 mol/h at T< 366K, rxn rates exp with T but not FT0 -r A (mol/gcat s) ext diff lim Variation of Reaction Rate with: Superficial velocity Superficial velocity Variation of Reaction Rate with: Type of Limitation Limitation Type of Particle size Particle size Temperature Temperature U1/2 U1/2 dp-3/2 dp-1 dp-1 dp-3/2 Linear Linear External External Independent Independent Exponential Exponential Internal Internal Independent Independent Independent Independent Exponential Exponential Surface reaction Surface reaction For FT0 = 100, 1000 & 5000 mol/h at T< 360K surface reaction limited For conditions FT0 = 1000 & 5000 mol/h at T< 366K, rxn rate is independent of FT0 but exponentially dependent on T surface reaction limited Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-26 The catalytic reaction A B takes place in a fixed bed reactor containing spherical porous catalyst X22. The overall rxn rates at a point in the reactor are shown in the graph below. For which, if any, of the conditions shown (flow rates and temps) is the reaction limited by internal diffusion? Rxn rates exp with T but not FT0 for FT0= 1000 & 5000 mol/h at T> 367K -r A (mol/gcat s) ext diff lim Internal diffusion limited when r Aincreases exponentially with T & is independent of velocity Variation of Reaction Rate with: Superficial velocity Superficial velocity Variation of Reaction Rate with: Type of Limitation Limitation Type of Particle size Particle size Temperature Temperature U1/2 U1/2 dp-3/2 dp-3/2 dp-1 dp-1 Linear Linear External External Independent Independent Exponential Exponential Internal Internal Independent Independent Independent Independent Exponential Exponential Surface reaction Surface reaction For FT0 = 1000 & 5000 mol/h at T> 367K, rxn rate is roughly independent of FT0 but exponentially dependent on T. The reaction rate is internal diffusion limited at T> 370K for FT0 = 1000 & 5000 mol/h Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-27 The catalytic reaction A B takes place in a fixed bed reactor containing spherical porous catalyst X22. The overall rxn rates at a point in the reactor are shown in the graph below. For which, if any, of the conditions shown (flow rates and temps) is the reaction limited by internal diffusion? -r A (mol/gcat s) ext diff lim Internal diffusion limited when r Aincreases exponentially with T & is independent of velocity Variation of Reaction Rate with: Superficial velocity Superficial velocity Variation of Reaction Rate with: Type of Limitation Limitation Type of Particle size Particle size Temperature Temperature U1/2 U1/2 dp-3/2 dp-1 dp-1 dp-3/2 Linear Linear External External Independent Independent Exponential Exponential Internal Internal Independent Independent Independent Independent Exponential Exponential Surface reaction Surface reaction How do we know it s not surface rxn limited at FT0=1000 & 5000 mol/h & T>367K? As T , the specific rate constant k , the rate of the surface rxn & consumption of reactant . Thus the reactant is more likely to be consumed before it reaches the core. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-28 The catalytic reaction A B takes place in a fixed bed reactor containing spherical porous catalyst X22. The overall rxn rates at a point in the reactor are shown in the graph below. For a flow rate of 10 g mol/h, determine the overall effectiveness factor at 360K -r A (mol/gcat s) actualoverallrxn rate = rxn rateif entireinterior surface were exposed to thebulk conditions Rxn w/out diffusion limitations r 0.70 =0.26 A = = 0.37 Ab r What do we use for the rate of reaction if the interior was exposed to bulk conditions? Use the rxn rate obtained under surface reaction limited conditions Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-29 The catalytic reaction A B takes place in a fixed bed reactor containing spherical porous catalyst X22. The overall rxn rates at a point in the reactor are shown in the graph below. For FT0= 5000 g mol/h, estimate the internal effectiveness factor at 367K -r A (mol/gcat s) actualoverallrate of reaction = rate of rxnif entireinterior surface were exposed to the external surface conditions Rxn w/out internal diffusion limitations 0.86 = r'' r'' 1.4 =1.2 A = As What do we use for the rate of reaction if the interior was exposed to the conditions at the surface of the pellet? Extrapolate the line for the surface reaction limited regime of the FT0 = 5000 mol/h plot to estimate the rxn rate that would be obtained without internal diffusion Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-30 Cl2 is removed from a waste stream by passing the effluent gas over a solid granular absorbent in a tubular PBR. Presently 63.2% is removed and the reaction is external diffusion limited. If the flow rate were increases by a factor of 4, the particle diameter were decreased by a factor of 3, and the tube length (z) were increased by 1.5x, what percentage of Cl2 would be removed (assume still external diffusion limited)? What guidelines (T, CA, ) do you propose for efficient operation of this bed? XA1=0.632 for dp, z, & XA2=? for dp1/3, 1.5z1, and 4 Variation of Reaction Rate with: Superficial velocity Type of Limitation Particle size Temperature U1/2 dp-3/2 dp-1 Linear Exponential Exponential External Internal Surface reaction Independent Independent Independent Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-31 Cl2 is removed from a waste stream by passing the effluent gas over a solid granular absorbent in a tubular PBR. Presently 63.2% is removed and the reaction is external diffusion limited. If the flow rate were increases by a factor of 4, the particle diameter were decreased by a factor of 3, and the tube length (z) were increased by 1.5x, what percentage of Cl2 would be removed (assume still external diffusion limited)? What guidelines (T, CA, ) do you propose for efficient operation of this bed? XA1=0.632 for dp, z, & XA2=? for dp1/3, 1.5z1, and 4 Need to relate XA to reactor length in the presence of an external diffusion limit Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Review: Mass Transfer Limited Rxn in PBR L21b-32 b a c a d a + + A B C D A steady state mole balance on reactant A between z and z + z : ( ) 6 1 + = = Az z F Az z F r'' a (A z) 0 where a + z A c c c d p ac: external surface area of catalyst per volume of catalytic bed (m2/m3) : porosity of bed, void fraction r A: rate of generation of A per unit catalytic surface area (mol/s m2) Divide out Ac z: c A z Put Faz and rA in terms of CA: Axial diffusion is negligible compared to bulk flow (convection) F B A UC A = = z z Substitute into the mass balance ( ) A A c r'' a 0 dz dz dp: particle diameter (m) dF dz 1 Az z F Az z F Take limit as z 0: W = z Az + = + r'' a 0 z + = r'' a 0 A c A c A = c + F A (J B )A A A c A A c z z z A A c A c dC dz dC dU dz d UC 0 A A + = + + = U r'' a 0 U C r'' a 0 + = A c A A c Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Review: Mass Transfer Limited Rxn in PBR (continued) b A B a At steady-state: Molar flux of A to particle surface = rate of disappearance of A on the surface r'' W k = = mass transfer coefficient kc =DAB/ (s-1) boundary layer thickness CAs: concentration of A at surface ( ) c c A As U k a C C 0 dz dC U k a C 0 dz varies with distance down reactor dC U k a C dz A C A0 k a C exp z C U L21b-33 c a d a dC dz A + + + = C D U r'' a 0 A c ( ) C C Substitute A Ar c A As CA: concentration of A in bulk dC A = CAs 0 in most mass transfer-limited rxns Rearrange & integrate to find how CA and the r A A = c c A C c c k a U C C z c c k a U dC C A A = A A ln z = = dz c c A A0 0 c c k a U c c k a U c c A = = = C C exp z r'' k C exp z A A0 A c A0 A0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-34 Cl2 is removed from a waste stream by passing the effluent gas over a solid granular absorbent in a tubular PBR. Presently 63.2% is removed and the reaction is external diffusion limited. If the flow rate were increases by a factor of 4, the particle diameter were decreased by a factor of 3, and the tube length (z) were increased by 1.5x, what percentage of Cl2 would be removed (assume still external diffusion limited)? What guidelines (T, CA, ) do you propose for efficient operation of this bed? XA1=0.632 for dp, z, & XA2=? for dp1/3, 1.5z1, and 4 ac: external surface area of catalyst per catalyst bed volume : porosity of bed ( ln 1 X ( ) For an external diffusion limited rxn in a PBR, we found (L19): 6 1 c c k a U C C A = = a exp z c d p A0 ( ) C 1 X C c c k a U c c k a U In terms of XA: ) A0 A = = exp z z A A0 ( ( ) ) ln 1 X ln 1 X c1 c1 k a zU a Express XA at 2 reaction conditions as a ratio: ( 6 1 d a 6 1 a d Relate U to & ac to dp A1 2 = ( ) k 1.5z U A2 c2 c2 1 ) = A A U= A where A U U cross-sectional area of PBR U U 0 c c p1 d c1 a a = p2 d 0,1 c 0,1 1 1 c1 = = ( ) = c2 2 0,2 c 2 0,2 c2 p1 p2 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-35 Cl2 is removed from a waste stream by passing the effluent gas over a solid granular absorbent in a tubular PBR. Presently 63.2% is removed and the reaction is external diffusion limited. If the flow rate were increases by a factor of 4, the particle diameter were decreased by a factor of 3, and the tube length (z) were increased by 1.5x, what percentage of Cl2 would be removed (assume still external diffusion limited)? What guidelines (T, CA, ) do you propose for efficient operation of this bed? XA1=0.632 for dp, z, & XA2=? for dp1/3, 1.5z1, and 4 ( ( ) ) d ln 1 X ln 1 X c1 c1 k a zU a U U a a How are kc1 and kc2 related? 0,1 p2 d A1 2 1 c1 = = = ( ) k 1.5z U A2 c2 c2 1 2 0,2 c2 p1 1 2 13 Ud D p AB d Typically the 2 is negligible so = + k 2 0.6 c D p AB 2 3 1 2 1 2 1 3 D U d U d D A B p = ( ) k 0.6 AB d = k 0. 6 c c 1 6 1 2 D 1 2 1 1 2 p,1 1 2 2 1 2 p,2 d p p A B U d 2 3 D AB 1 6 1 2 1 2 1 1 2 p,1 d k k p,2 d U 0.6 0.6D k k U c1 = c1 = 2 3 1 2 2 U c2 AB 1 6 c2 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-36 Cl2 is removed from a waste stream by passing the effluent gas over a solid granular absorbent in a tubular PBR. Presently 63.2% is removed and the reaction is external diffusion limited. If the flow rate were increases by a factor of 4, the particle diameter were decreased by a factor of 3, and the tube length (z) were increased by 1.5x, what percentage of Cl2 would be removed (assume still external diffusion limited)? What guidelines (T, CA, ) do you propose for efficient operation of this bed? XA1=0.632 for dp, z, & XA2=? for dp1/3, 1.5z1, and 4 ( ( ) ) ( ( ) ) ln 1 X ln 1 X ln 1 X ln 1 X c1 c1 k a zU a k k a a U z A1 A1 c1 c1 2 2 = = ( ) 1.5z U k 1.5z U A2 c2 c2 1 A2 c2 c2 1 1 2 d 1 2 1 1 2 p,1 d a a U U p,2 d U k k U p2 d 0,1 c1 1 = = c1 = 1 2 2 c2 p1 2 0,2 c2 1 2 ( ( ) ) 1 2 1 1 2 p 1 , d ) ) p,2 d d ln 1 X ln 1 X U z p 2 0,2 A1 = 1 2 2 U d 1.5z A2 p1 0,1 3 2 3 2 3 2 1 2 ( ( ) ) ( ( 1 2 1 1 2 2 U d p,2 d d ln 1 X l n 1 X ln 1 X ln 1 X U 1 1 p,2 d 0,2 0,1 0, 2 A1 A1 = = 3 2 1 2 3 2 1.5 1.5 A 2 0,1 A2 0,1 p,1 0,2 p,1 ( ) 1 2 12 3 2 ( ) ( ( ) ) 4 d 3 p,2 d d ln 1 ln 0.632 1 X 1 ln 1 X ln 1 X 1 0,1 p,1 d 0,2 A1 = = ( ) 1 2 3 2 1. 5 12 3 2 1.5 A2 0,1 p,1 A2 0,1 p,1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-37 Cl2 is removed from a waste stream by passing the effluent gas over a solid granular absorbent in a tubular PBR. Presently 63.2% is removed and the reaction is external diffusion limited. If the flow rate were increases by a factor of 4, the particle diameter were decreased by a factor of 3, and the tube length (z) were increased by 1.5x, what percentage of Cl2 would be removed (assume still external diffusion limited)? What guidelines (T, CA, ) do you propose for efficient operation of this bed? XA1=0.632 for dp, z, & XA2=? for dp1/3, 1.5z1, and 4 ( ) 3 2 1 2 ( ) ( ) 4 d 3 ln 1 0.632 ln 1 X ln 1 0.632 ln 1 X 1 )3 2 0,1 p,1 d ( = 2 1 3 0.667 = ( ) ( ) 1 2 3 2 1.5 A2 A2 0,1 p,1 ( ) 0.99967 ln 1 X ln 0.368 ln 1 X = 0.257 = 0.257 ( ) ( ) A2 A2 ( ) 3.8898 1 X = = 0.0204 3.8898 ln 1 X 1 X = e A2 A2 A2 = X 0.98 A2 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-38 Cl2 is removed from a waste stream by passing the effluent gas over a solid granular absorbent in a tubular PBR. Presently 63.2% is removed and the reaction is external diffusion limited. If the flow rate were increases by a factor of 4, the particle diameter were decreased by a factor of 3, and the tube length (z) were increased by 1.5x, what percentage of Cl2 would be removed (assume still external diffusion limited)? What guidelines (T, CA, ) do you propose for efficient operation of this bed? XA1=0.632 for dp, z, & XA2=0.98 for dp1/3, 1.5z1, and 4 Hint for T: The conversion of 0.98 is dependent on the reaction still being external diffusion-limited. How can we adjust the T, CA, and to make sure that the process is not instead slowed down by the surface reaction, but without slowing down external diffusion? Variation of Reaction Rate with: Superficial velocity Type of Limitation Particle size Temperature U1/2 dp-3/2 dp-1 Linear Exponential Exponential External Internal Surface reaction Independent Independent Independent To keep the rate of Cl2 consumption (surface reaction) faster than external diffusion (still in external diffusion limited regime), use high T Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L21b-39 Cl2 is removed from a waste stream by passing the effluent gas over a solid granular absorbent in a tubular PBR. Presently 63.2% is removed and the reaction is external diffusion limited. If the flow rate were increases by a factor of 4, the particle diameter were decreased by a factor of 3, and the tube length (z) were increased by 1.5x, what percentage of Cl2 would be removed (assume still external diffusion limited)? What guidelines (T, CA, ) do you propose for efficient operation of this bed? XA1=0.632 for dp, z, & XA2=0.98 for dp1/3, 1.5z1, and 4 Hint: How does changing CA and influence the rate of external diffusion and the surface reaction? The mass transfer rate can be increased by increasing the concentration gradient, which is achieved by increasing the bulk concentration of A ( ) 1 2 2 3 A D 0,1 c AB 1 6 = k 0.6 c 1 2 p d Increasing the volumetric flow rate increases the mass transfer coefficient but reduces the spacetime, and therefore XA. The process also becomes reaction limited instead of external diffusion limited. XA,mass x-fer kc but XA,reaction so the increase in may be offset by a reaction-limited decrease in conversion, assuming constant packed-bed properties. We would need the parameters for the reaction to evaluate whether increasing is a good idea. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.