Understanding Stoichiometry in Chemistry
Explore the concept of stoichiometry in chemistry, which involves quantitative relationships in balanced chemical reactions. Learn about moles, mole ratios, and mass-to-mass calculations through practical examples and problem-solving methods.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chemistry Mar 27, 2017 Get out Chemical Formula Worksheet for HMK check P3 Challenge Determine the number of Mg atoms in 0.35 g of MgO. Turn in Xtra Credit assignments. (NO late accepted.) Today s Objective Stoichiometry Agenda Hmk Review Moles and Reactions Assignment: Stoichiometry Worksheet
Gram mole number of items problems Not Here!! Grams of X Grams of Y Molar mass Molar mass Chemical formula Moles of X Moles of Y Macroscopic scale 6.022x1023 6.022x1023 Chemical formula Number of X Number of Y Atomic scale
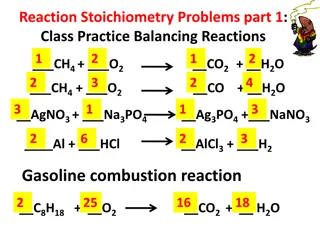
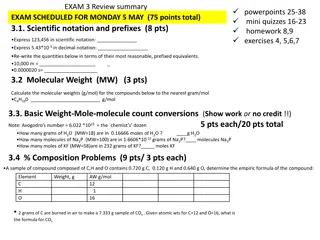
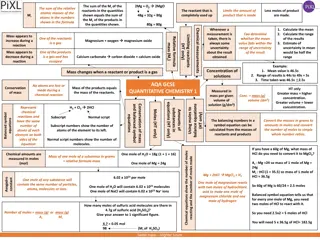
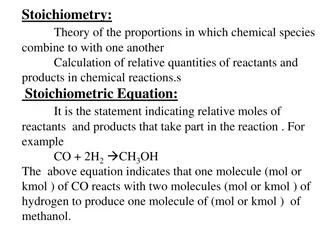
Stoichiometry Stoichiometry is the study of the quantitative relationships present within a balanced chemical reaction A balanced reaction has the same number of atoms of each element on both sides to achieve conservation of mass. The coefficients tell how many formula units are required or produced to achieve this. Because a mole is simply a way to count items, the coefficients of a balanced reaction also relate the number of moles of reactants and products.
Mole Ratios Consider 1 Zn (s) + 2 HCl (aq) 1 ZnCl2 (aq) + 1 H2 (g) Several mole ratios are possible. These are just some: 1 mol ZnCl 1 mol H 1 mol ZnCl 1 mol Zn 2 mol HCl 1 mol H 1 mol Zn 1 mol H 1 mol Zn 2 mol HCl 2 mol HCl 1 mol ZnCl 2 2 2 2 2 2
Stoichiometry Problem Solving Same problem solving. Only difference is you need a balanced reaction first. 1 ZnCl2 (aq) + 1 H2 (g) Ex: How many moles of hydrogen are formed from 3.50 mole of hydrochloric acid? 1 Zn (s) + 2 HCl (aq) 1 mol H 2 mol HCl = 3.50 mol HCl 1.75 mol H 2 2
Gram mole number of items problems Not Here!! Grams of X Grams of Y Molar mass Molar mass Chemical formula Moles of X Moles of Y Macroscopic scale 6.022x1023 6.022x1023 Chemical formula Number of X Number of Y Atomic scale
Stoichiometry Not Here!! Grams of X Grams of Y Molar mass Molar mass Balanced Reaction Moles of X Moles of Y Macroscopic scale 6.022x1023 6.022x1023 Balanced Reaction Number of X Number of Y Atomic scale
Stoichiometry Mass to mass calculations most common, or a subset gram mole mole gram Information Needed A) the balanced equation B) the molar masses of the reactants and products involved
Stoichiometry g-m-m-g Problem How many grams of lithium hydroxide are produced when 25.0 g of lithium is dissolved in water? 2 LiOH + 1 H2 25.0 g g ? 2 Li + 2 H2O gram mole mole gram 1 mol Li 6.94 g Li 2 mol LiOH 23.95 g LiOH 2 mol Li = 25.0 g Li 86.31 g LiOH 1 mol LiOH
Stoichiometry g-m-m Problem How many moles of lithium are needed to produce 25.0 g of lithium hydroxide dissolved in water? 2 LiOH + 1 H2 mol? 25.0 g mole mole gram 2 Li + 2 H2O gram 1 mol LiOH 23.95 g LiOH 2 mol LiOH 2 mol Li = 25.0 g LiOH 1.04 mol Li
Stoichiometry How many grams of potassium chlorate do you need to decompose to produce 3.75 moles of oxygen gas? 2 KCl + 3 O2 g? 3.75 mol 2 KClO3 mole mole gram 2 mol KClO 3 mol O 122.55 g KClO 1 mol KClO = 3.75 mol O 306 g KClO 3 3 2 3 2 3
Exit Slip - Homework Exit Slip: 2 KClO3 How many grams of oxygen are produced from 15.0 g of KClO3? 2 KCl + 3 O2 What s Due? (Pending assignments to complete.) Stoichiometry worksheet What s Next? (How to prepare for the next day) Read p100-102, p224-232, 241 p246-247