Understanding the Basicity of Amines with Dr. Sasmita Panda
Explore the concept of amine basicity with Dr. Sasmita Panda from the Department of Chemistry at B.J.B. College, Bhubaneswar. Dive into the properties and reactivity of amines to enhance your understanding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
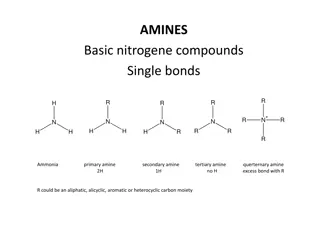
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES Ortho effect in substituted aniline (steric inhibition of protonation) When any group is present at ortho to NH2 in aniline then the basic character of that compound becomes at least less than aniline. For example, see the order of basicity of following substituted aniline:- p-Toluidine > m-Toluidine > Aniline > o-Toluidine Aniline > m-Nitroaniline > p-Nitroaniline > o-Nitroaniline Aniline > p-Haloaniline > m-Haloaniline > o-Haloaniline p-Aminophenol pKb=8.50 > o-Aminophenol pKb=9.28 > Aniline pKb=9.38 > m-Aminophenol pKb=9.80 DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES The protonation of Substituted aniline is inhibited due to steric hindrance. Upon protonation the hybridization of Nitrogen in amino group changes from sp2 to sp3 making the group non planar. This causes the steric hindrance between the ortho substituted group and H atom of amino group which makes the conjugate acid less stable, hence decreases the basicity of substituted aniline DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
BASICITY OF AMINES DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis The Gabriel synthesis or Gabriel phthalimide synthesis is named after the German chemist Siegmund Gabriel. Gabriel phthalimide synthesis is a reaction that involves conversion of primary alkyl halides into primary amines using alkyl halides. Conventionally, the Gabriel synthesis uses potassium phthalimide. DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis The Gabriel phthalimide synthesis or Gabriel synthesis has applications in the alkylation of sulfonamides and imides, followed by their deprotection, to obtain amines. In the Gabriel phthalimide synthesis, phthalimide anion is recruited as a proxy of H2N . The main objective of Gabriel phthalimide synthesis is to form primary amine (RNH2). Gabriel synthesis involves reaction of potassium hydroxide with the phthalimide which forms a good nucleophile in the form of an imide ion. The imide ion attacks alkyl halide via nucleophilic substitution reaction and leads to the formation of an intermediate named as N-alkyl phthalimide. Phthalimide then undergoes hydrolysis which yields a primary alkyl amine. Aryl amines cannot be prepared through Gabriel synthesis because aryl halides do not undergo simple nucleophilic substitution. DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis Mechanism The reaction initiates with conversion of phthalimide into potassium phthalimide by addition of alkali base KOH or NaOH. Removal of protons from the nitrogen of phthalimide takes place; deprotonation of nitrogen The nitrogen of potassium phthalimide gains a negative charge due to deprotonation and act as a nucleophile and react with alkyl halide via SN2 reaction. DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis The nucleophilic nitrogen of Gabriel phthalimide attacks the electrophilic carbon of alkyl halide. The potassium ion from KOH combines with halogen of alkyl halide and forms KX. The alkyl group of alkyl halide attaches to nucleophilic nitrogen which results in the formation of N-alkyl phthalimide DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis Hydrazine (NH2NH2) is used via the Ing Manske procedure, produces a precipitate of phthalhydrazide along with the primary amine. Processes like acidic hydrolysis or basic hydrolysis can sometimes be done. Gabriel synthesis involving acidic hydrolysis liberates amine salt from the primary amine. DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis The nitrogen of hydrazine is deprotonated by nitrogen of phthalimide which leads to the formation of an uncharged species. DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis Unreacted NH2 group of hydrazine undergoes nucleophilic acyl substitution and attacks the carbonyl group liberating amine. DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis The final step of Gabriel phthalimide reaction involves neutralization of charge which results in formation of primary amine RNH2. This step marks the end of Gabriel phthalimide synthesis. DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis Gabriel phthalimide synthesis involving basic hydrolysis Attack of OH- on one of the carbonyl carbon by nucleophilic substitution reaction. Cleaving of N-alkyl phthalimide. During this process the oxygen atom of the carbonyl group gains a negative charge. Another molecule of OH ion attacks the second carbonyl group. This process of charge transfer between nucleophile and electrophile to gain stability and neutralize molecules forces RNH2 to detach from carbonyl carbon. The nitrogen is replaced by O- ions. This marks the end of nucleophilic substitution reaction and hence end of Gabriel phthalimide synthesis. DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR
Gabriel Phthalimide synthesis DR. SASMITA PANDA DEPARTMENT OF CHEMISTRY B. J. B. (AUTO) COLLEGE, BHUBANESWAR