Week 4: Compounds and Elements Test
In this week's science lesson, students focus on compounds and elements, conducting a density test and exploring the combination of atoms to form compounds. Topics include recognizing elements, molecules, and compounds through models and symbols. The lesson also covers the concept that all living and nonliving things are made up of compounds. Students engage in activities, quizzes, and a chemical reaction modeling exercise.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Week 4 September 5-9 Compounds and Elements
Objective Today I will complete my test on density 2. So that I can demonstrate my gains on bench march SC.8.P.8.3 on density. I know I have it when I can pass my test 9 out of 13.
Density 1 SC.8.P .8.3 C= 0 H= none A= Test M= none, I will collect test P=Looks like students writing down school wide heading and bubbling I.D. number. Set folders up and don t write on test. Wait until test has been picked up. S=9 out of 13
Benchmark SC.8.P.8.8 Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter. Know Understand Atom Element Molecules Compound Models Compounds make up non living things Do We have a finite of elements Atoms combine in multiple ways to form compounds Compounds make up all living things Quick notes and practice Study Jam elements and compounds Lab Demo sulfur and copper Homework Quiz 7/7 notes
Which of the following describes both of the substances in her model? A. They cannot be combined together to make new substances. B. They can be broken down into new substances by physical means. C. They cannot be broken down into simpler substances by chemical means. D. They can only combine together in one way to make a new substance.
Quick Notes: Champs SC.8.P .8.5 Today I will look for text evidence and symbols that model elements and compounds. So that can explain what an element, molecule and compound are. Recognize models of element, molecules and compounds I know I have it when I have completed my questions. I can answer my exit question with 100 percent accuracy.
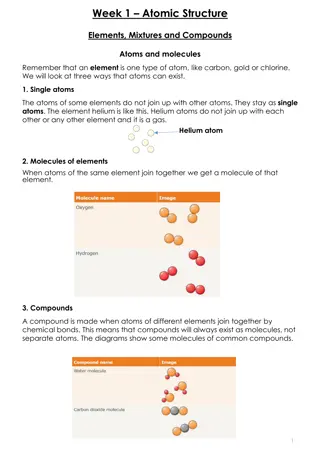
Quick Notes SC.8.P.8.5 Models Models can be used to represent elements and compounds. Everything is made up of atoms of elements except the electromagnetic spectrum. Many compounds can be made by different combinations of the same elements.
Alisa is making a model of a chemical reaction. She uses different color spheres to represent atoms of elements. Her model is shown below. Which of the substances shown in Alisa s model are compounds? A A and B B and C A. B. C. D. C
Go to page 218 # your page 1-3 and justify 1. B-only bonds and combinations can be illustrated in the model not thermal energy. 2. G-Figure B because they are not all joined together. 3. D-because the models are smaller than the other it would have to be less dense.
Components of Matter page 190 Explain what an atom is? Atom is the basic unit from which all matter is made. What happens when different atoms combine? Different substances are made. Look at page 191 and count the number of atoms for the below chart. Element Type of atom Type of atom oxygen 2 oxygen Carbon dioxide 2 oxygen Methane 1 carbon 4 hydrogen 1 carbon
Compounds of Matter Elements p.190 and Molecules p.191 What is an element? Elements are made up only one type of atoms. Provide an example of an element in the text. Aluminum is an example because aluminum is only made of one type of atom. What is a molecule? When atoms combine with other atoms it forms a molecule.
Compounds of Matter Molecules p.191 What is a chemical bond? The force of attraction between two or more atoms which form molecules. What are examples of molecules based on the text? Carbon dioxide and hydrogen atoms can combine together.
Compounds of Matter Compounds p.192 Write sentence out and fill in the blank When a molecule contains more than __________ it is called a compound. Compounds are made of two elements that are chemically combined in a set _________ Compounds are represented a chemical ______ What is the chemical formula for carbon dioxide? ________ The small number 2 is called a _________. Element ratio formula Chemical formula subscript
One of the products of photosynthesis is the sugar glucose, represented by the chemical formula C6H12O6.Which is true of glucose molecules? A. Each molecule is chemically identical. B. Each molecule contains a single nucleus surrounded by an electron cloud. C. Each molecule contains 6 hydrogen atoms and 12 oxygen atoms. D. Each molecule is made up of glucose atoms joined by chemical bonds.
Which of these particles is thesmallest? A. an atom B. a nucleus C. a proton D. an electron
A substance contains an arrangement of different types of atoms joined together by chemical bonds. Which of the following classes of substances could this describe? A. all matter B. all elements C. all compounds D. all pure substances
Quick Notes Match A Elements cannot be broken down into 1 as an element or compound. B Many compounds can be made C Particles (atoms) that make 2 than the elements that make them up. 3 simpler substances by chemical means. A-3 B-4 C-5 D-1 E-2 D Substances can be classified as 4by different combinations of the same elements. EUnderstand compounds have unique an distinct properties different 5 up an element must always be the same.
Jorgen takes a drink of water to cool down after playing basketball. What do Jorgen, the basketball, and the water all have in common? A. They are made of atoms of elements. B. They are made of the same compounds. C. They are each made of one type of element. D. They are each made of one type of compound.
Bao has a sample of graphite, which is made of carbon. She learns that diamond and carbon nanotubes are also made of carbon. What can she conclude about carbon nanotubes, diamond, graphite? A. They have the same properties. B. The atoms in all three substances are the same. C. They can be broken down into simpler substances by chemical means. D. The carbon atoms changed into different elements to form the different substances.
Study Jam Elements and Compounds http://studyjams.scholastic.com/studyjams/jams/science/matter/elem ents-and-compounds.htm C=0 during instruction and when students share H=ask teacher A= Study Jam Video M=none P=Looks like students writing down 2 column notes and answering questions. Looks like students summarize finding and sharing with a partner. S=Check for success.
Name Date Period Page Study Jam Elements and compounds Essential Questions: What is the main ingredient to form compounds Questions 1. Infer how elements are like an ingredient of a cake? 2. When 2 or more elements combine chemically what if formed? 3. What are elements? 4. When elements combine chemically we can write them in a _____________ 5. What is an example of a chemical formula What have you learned so far: Notes and Evidence 1. Elements combine to form all the substances in the universe 1. Compound 2. Substances that cannot be broken down into something simpler 3. Chemical formula 4. H2O which is water
Name Date Period Page Study Jam Elements and compounds Essential Questions: What is the role of a chemical formula? Questions 1. What is a subscript? 2. What is the chemical formula salt? 3. How many elements are listed on the periodic table? Notes and Evidence 1. That is the small number on the right that tells you the number of atoms it has. 2. NaCl 3. 118 Summarize what you learned: