Chemical Kinetics Study: Factors, Reactions, and Models
Delve into the realm of chemical kinetics with a focus on factors influencing reaction rates, basic reaction principles, the Law of Mass Action, homogeneous reactions, and derivation of key equations. Explore the significance of concentration, temperature, pressure, surface area, catalysts, and inhibitors in chemical reactions. Discover reaction models and their mathematical formulations in this insightful guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Boom! Chemical Kinetics Anthony Doria MAT 493 5/6/2015
Outline Introduction Homogeneous Case Non-Homogeneous Case Semi-Linear Case
What is a Chemical Reaction? A process that transforms one or more substances into another. Examples: Photosynthesis Lighting a match Rusting
Chemical Kinetics Chemical kinetics is the study of the rates of chemical reactions. Analyze how factors of interest influence the reaction rates
Factors of Interest Concentration Temperature Pressure Surface Area Catalysts Inhibitors
A Basic Reaction A the reactant B the product k the rate of reaction (comes from the Law of Mass Action) ?? ?
Law of Mass Action Formulated by Guldberg and Waage The rate of any chemical reaction is proportional to the product of the masses of the reacting substances, with each mass raised to a power equal to the coefficient that occurs in the chemical reaction ( Law of Mass Action ).
Law of Mass Action Mathematically, ?1 ?? + ?? ?? + ?? ?2 =[?]?[?]? [?]?[?]? ? =?1 ?1[?]?[?]?= ?2[?]?[?]? ?2
Two Reaction Models Two-step sequence first order reaction model ?? ?? ? Two-step sequence first order reaction model with back reactions ?1 ?1 ? ? ? ?2 ?2
Deriving the Equations Think rate in rate out For (2-SFOM), the equations are: ? ? ?? = ? ? ? ? ?? = ? ? ? ? ? ? ?? ? 0 = ?0 ? 0 = ?0 ? 0 = ?0 = ? ?
HCP For (2-SFOM), HCP is: The model can be written in a compact vector form, known as HCP form: ? ? ? ? ? ? ? ? 0 0 0 0 ? ? ? = ?? ? ,? > ?0 ? ?0 = ?0 ? = ? 0 ?0 ?0 ?0 ? ? ? 0 =
Solution and Continuous Dependence By EXPLORE! (118-120), as long as the errors are small, the perturbed version of (2-SFOM) will behave like the original. Theorem 4.6.1: HCP has a unique solution given by: ? ? = ??(? ?0)?0
Catalysts Speed up the rate of reaction without directly entering into the reaction The new (2-SFOM) model: ?? ?? ? + ?
Non-CP The new models can be written in this form. Theorem 5.3.1 is applied, so each model has a unique solution. EXPLORE! (145-147) results are applied, as long as the errors are small, the perturbed version will behave like the original. Non-CP form: ? ? = ?? ? + ?(?),? > ?0 ? ?0 = ?0
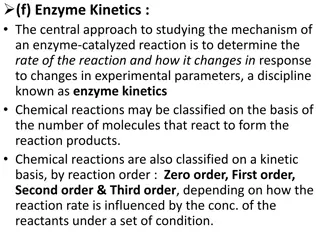
Enzymes Chemical reactions occur in all living things. But, most of these reactions take too long to occur Enzymes are proteins that speed up chemical reactions within the cell
How Enzymes Work The enzyme and the substrate combine This forms an enzyme-substrate complex. The complex forms the product When the product is released, the enzyme remains unaltered and repeats this process. Modeled by Michaelis and Menten
Michaelis-Menten Enzyme Kinetics Model ?1 ? + ? ?? ? 1 ?2? + ? ?? ?3 ? + ? ?? ? 3 ?4? + ? ??
Michaelis-Menten Enzyme Kinetics Model When broken down, it cannot be written in Semi-CP form. There is no easy way to define the A matrix (see below) ?[?] ?? [?]0[?] = ?2 ? 1+ ?2 ?1 [?]0[?] ? 1+ ?2 ?1 + [?] ?[?] ?? = ?2 + [?]
Taylor to the Rescue! However, we can use a Taylor approximation of (1 + x)-1to write the model in Semi-CP form: ?[?] ?? ?[?] ?? ? ? = ? (1 ??) ? ??) ?? ? ?? = ? (1 Theorem 7.6.1 can then be applied, and a solution exists for the model
Some Extra Information All the models presented here were of first order. Reactions can be as high as 2nd, 3rd or even 4th order. The models here generally consisted of only one reactant transforming into one product. Some reactions need multiple reactants to occur They might also create more than one product
References "Law of Mass Action." Encyclopedia Britannica Online. Encyclopedia Britannica, n.d. Web. 15 Mar. 2015.