Comparison of PI vs. PI: ATV vs. ATV/r, LPV/r mono vs. LPV/r + ZDV/3TC
Examination of protease inhibitor regimens, including atazanavir and lopinavir/ritonavir, in mono or combination therapies, along with baseline characteristics, patient response at week 48, virologic failure rates, and resistance data.
Uploaded on Apr 16, 2025 | 2 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
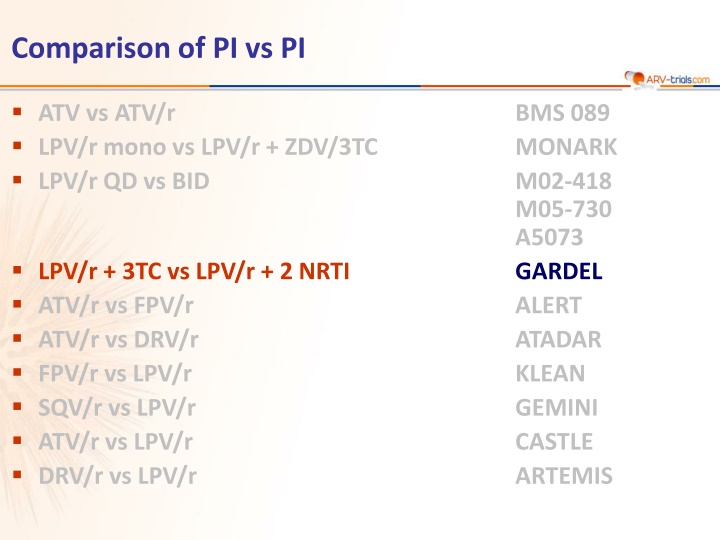
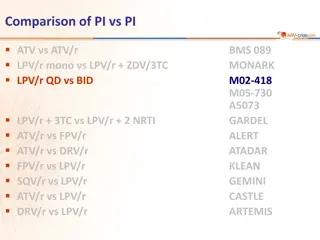
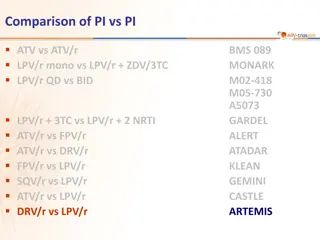
Comparison of PI vs PI ATV vs ATV/r LPV/r mono vs LPV/r + ZDV/3TC LPV/r QD vs BID BMS 089 MONARK M02-418 M05-730 A5073 GARDEL ALERT ATADAR KLEAN GEMINI CASTLE ARTEMIS LPV/r + 3TC vs LPV/r + 2 NRTI ATV/r vs FPV/r ATV/r vs DRV/r FPV/r vs LPV/r SQV/r vs LPV/r ATV/r vs LPV/r DRV/r vs LPV/r
GARDEL Study: LPV/r + 3TC vs LPV/r + 2 NRTI Design Randomisation* 1 : 1 Open-label W48 W96 N = 217 > 18 years ARV-na ve LPV/r 400/100 mg + 3TC 150 mg BID HIV RNA > 1,000 c/mL Any CD4 cell count HBsAg negative No R to study drugs N = 209 LPV/r 400/100 mg BID + FDC of 2 NRTI** *Randomisation was stratified by HIV RNA (<or > 100,000 c/mL) at screening ** Investigator-selected NRTI : ZDV/3TC = 54%, TDF/FTC = 37%, ABC/3TC = 9% Objective Non inferiority of LPV/r + 3TC at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (lower limit of the 95% CI for the difference = -12%, 85% power) Cahn P. Lancet Infect Dis 2014;14:572-80 GARDEL
GARDEL Study: LPV/r + 3TC vs LPV/r + 2 NRTI Baseline characteristics and patient disposition LPV/r + 3TC N = 214 34 16% 4.87 44% 319 21% 16 (7.5%) N = 1 N = 1 N = 7 N = 5 N = 1 0 N = 1 LPV/r + 2 NRTI N = 202 35 17% 4.87 43% 329 19% 27 (13.4%) N = 6 N = 10 N = 9 N = 1 0 N = 1 0 Median age, years Female HIV RNA (log10 c/mL), median HIV RNA > 100,000 c/mL CD4 cell count (/mm3), median CD4 < 200 per mm3 Discontinuation by W48 For virologic failure at week 24 For adverse event Lost to follow-up Non-compliance Death Opportunistic infection Pregnancy Cahn P. Lancet Infect Dis 2014;14:572-80 GARDEL
GARDEL Study: LPV/r + 3TC vs LPV/r + 2 NRTI Response to treatment at week 48 HIV RNA < 50 c/mL All patients Primary analysis All patients Baseline HIV-1 RNA 100 000 c/mL % 96.6 100 95.5 LPV/r + 3TC LPV/r + 2 NRTI 88.3 87.2 83.7 77.9 75 50 Median CD4/mm3 increase : + 227 (LPV/r + 3TC) vs + 217 (LPV/r + 2 NRTI) 25 0 ITT, snapshot ITT, snapshot Observed Adjusted difference (95% CI)= 4.6% (- 2.2 ; 11.8) Adjusted difference (95% CI)= 9.3%(- 2.8 ; 21.5) Adjusted difference (95% CI)= - 1.1% (- 5.6 ; 3.4) Cahn P. Lancet Infect Dis 2014;14:572-80 GARDEL
GARDEL Study: LPV/r + 3TC vs LPV/r + 2 NRTI Virologic failure definition - 2 consecutive HIV-1 RNA > 400 c/mL at or after W24 - HIV-1 RNA > 50 c/mL at W48 Resistance data at week 48 LPV/r + 3TC LPV/r + 2 NRTI Virologic failure 10 12 At week 24 1 6 At week 48 9 6 Median HIV-1 RNA at virologic failure, c/mL 236 1027 Success of amplification for genotype testing 5/10 7/12 Presence of resistance mutations 2 0 M184V Protease inhibitor resistance 2 0 - - Cahn P. Lancet Infect Dis 2014;14:572-80 GARDEL
GARDEL Study: LPV/r + 3TC vs LPV/r + 2 NRTI Adverse events LPV/r + 3TC LPV/r + 2NRTI P Grade 2-3 AE possibly or probably drug related 65 (30%) 88 (44%) 0.007 Patients with grade 2-3 AE possibly or probably drug related 43 48 - Drug-related AE 2% in either group Hyperlipidaemia 23 16 - Diarrhoea 14 14 - Nausea 2 9 0.05 Dyspepsia 2 6 0.02 AE leading to discontinuation 2 (1%) 11 (5%)* 0.01 NRTI related - Zidovudine 0 11 10 (anaemia = 3, GI, N = 6, rash = 1) 1 (rash) - Tenofovir Selected grade 3-4 laboratory abnormalities occurred at the same frequency in both groups, except for hyperlipidemia (more frequent in dual therapy group) Cahn P. Lancet Infect Dis 2014;14:572-80 GARDEL
GARDEL Study: LPV/r + 3TC vs LPV/r + 2 NRTI Summary LPV/r + 3TC dual therapy was virologically non inferior to a standard therapy of LPV/r + 2 NRTI Similar virologic response of the 2 regimens in patients with HIV RNA > 100 000 c/mL at enrolment No resistance mutations to protease inhibitor at virologic failure in either group 2 patients with M184V in dual therapy group Incidence of adverse events higher in triple therapy group Discontinuation because of adverse events mainly related to NRTI in the LPV/r + NRTI arm Potential advantages of first-line LPV/r + 3TC Cost Less toxicity (might need less monitoring) Spares the other NRTIs Cahn P. Lancet Infect Dis 2014;14:572-80 GARDEL