LEAGUE-1 study: daclatasvir + SMV + RBV for genotype 1
The LEAGUE-1 study evaluated the efficacy of Daclatasvir (DCV), Simeprevir (SMV), and Ribavirin (RBV) for treating chronic HCV genotype 1 infection. The open-label, randomized trial involved treatment-naïve and prior null responders, stratifying participants based on cirrhosis status. Outcomes measured included SVR12 rates, treatment duration effects, and patient disposition across different genotypes. Results indicated high sustained virologic response rates, emphasizing the study's impact on treatment approaches for HCV.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
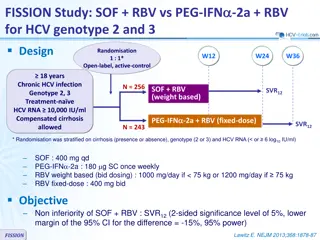
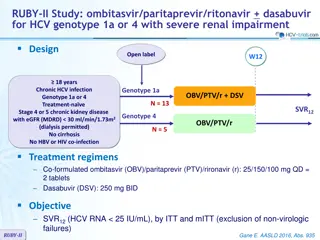
LEAGUE-1 study: daclatasvir + SMV + RBV for genotype 1 Design W12 W24 Randomisation* 1 : 1 Open label Randomisation 1 : 1 DCV 30 mg qd + SMV 150 mg qd N = 76 > 18 years Stop Chronic HCV infection Genotype 1 Treatment na ve or prior null response to PEG-IFN + RBV HCV RNA 100,000 IU/ml Compensated cirrhosis ** allowed No HBV or HIV coinfection GT 1b DCV 30 mg + SMV 150 mg qd + RBV Continue N = 71 N = 21 GT 1a DCV 30 mg qd + SMV 150 mg qd + RBV * Randomisation was stratified on cirrhosis (yes or no) and prior treatment (na ve or null response) ** Biopsy or Fibroscan 14.6 kPa RBV (bid dosing): 1000 mg/d (< 75 kg) or 1200 mg/d ( 75 kg) Objective SVR12(HCV RNA < 25 IU/ml), by mITT, with 80% CI, by cohort based on HCV genotype 1 subtype and prior treatment experience SVR12was also reported by treatment regimen (with or without RBV) and treatment duration (12 or 24 weeks) for the genotype 1b cohort Zeuzem S, J Hepatol 2016; 64:292-300 LEAGUE-1
LEAGUE-1 study: daclatasvir + SMV + RBV for genotype 1 Baseline characteristics and patient disposition Genotype 1b Genotype 1a Treatment-na ve Null responder DCV + SMV + RBV N = 51 DCV + SMV + RBV N = 20 DCV + SMV + RBV N = 21 * DCV + SMV N = 53 DCV + SMV N = 23 Median age, years 54 53 56 59 52 Female 59% 51% 48% 55% 33% Race : white 91% 92% 100% 85% 95% HCV RNA log10IU/ml, mean Metavir : F0-F2 / F3 / F4, % 6.2 6.4 6.4 6.3 6.2 70 / 19 / 11 77 / 10 / 14 39 / 22 / 39 60 / 20 / 20 43 / 29 / 29 IL28B CC 30% 26% 4% 5% 14% Resistance-associated polymorphisms NS5A NS3 14% 17% 22% 18% 33% 33% 20% 25% 0 48% Discontinuation, N Lack of efficacy Adverse event Other 7 4 2 1 8 5 2 1 6 4 0 2 1 1 0 0 13 11 0 2 * Treatment-na ve, N = 12 ; null responder to prior PEG-IFN + RBV, N = 9 Zeuzem S, J Hepatol 2016; 64:292-300 LEAGUE-1
LEAGUE-1 study: daclatasvir + SMV + RBV for genotype 1 SVR12(HCV RNA < 25 IU/ml) in genotype 1b DCV + SMV DCV + SMV + RBV % 100 100 100 93.8 95 88.7 88.9 84.9 85.3 85.1 80.8 83.3 83.3 78.6 82.6 75 74.5 73.9 75 69.6 57.8 55.6 50 25 9 N 53 51 6 7 47 44 4 14 16 23 20 0 12W of therapy 24W of therapy No 12W of therapy 24W of therapy No All Cirrhosis All Cirrhosis cirrhosis cirrhosis Treatment-na ve Null-responder Of the 29 failures, there were 15 virologic breakthrough and 5 relapses Zeuzem S, J Hepatol 2016; 64:292-300 LEAGUE-1
LEAGUE-1 study: daclatasvir + SMV + RBV for genotype 1 Outcome in genotype 1a SVR12in treatment-na ve patients: 66.7% All prior null responders with genotype 1a were offered the addition of PEG-IFN for 24 weeks after the initial 5 patients experienced virologic breakthrough. They were considered SVR12non responders Resistance analysis in genotype 1b At baseline, NS5A polymorphisms at L28, R30, L31, or Y93 were detected in 29/136 (21.3%) available sequences; SVR12in 16/29 (55.2%) compared with 97/107 (90.7%) patients without these polymorphisms Baseline NS3 polymorphisms at V36, T54, Q80, or S122 were detected in 30/138 genotype 1b infected patients with available sequences ; SVR12in 24/30 (80.0%) versus 89/108 (81.5%) patients without these polymorphisms Zeuzem S, J Hepatol 2016; 64:292-300 LEAGUE-1
LEAGUE-1 study: daclatasvir + SMV + RBV for genotype 1 Adverse events and grade 3-4 laboratory abnormalities, N (%) DCV + SMV N = 76 9.2% 2 (2.6%) 1 (1.3%) DCV + SMV + RBV N = 92 4.3% 2 (2.2%) 0 Serious adverse event Adverse event leading to discontinuation Death Adverse event in 10% of patients Asthenia Headache Nausea Pruritus Fatigue Nasopharyngitis Dyspnea Hemoglobin < 9 g/dl Neutrophils < 750/mm3 Lymphocytes < 500/mm3 ALT > 5 x ULN / AST > 5 x ULN Total bilirubin > 2.5 x ULN Lipase > 3 x ULN 55% 43% 35% 31% 34% 35% 28% 0 1 0 0 / 0 3 0 54% 43% 40% 34% 25% 34% 27% 0 0 1 1 / 1 14 1 Zeuzem S, J Hepatol 2016; 64:292-300 LEAGUE-1
LEAGUE-1 study: daclatasvir + SMV + RBV for genotype 1 Summary With all-oral, IFN-free treatment of DCV + SMV, with or without RBV In genotype 1b, treatment for 12 or 24 weeks achieved combined SVR12rates of 84.9% (DCV + SMV) and 74.5% (DCV + SMV + RBV) in treatment na ve patients and 69.6% (DCV + SMV) and 95.0% (DCV + SMV + RBV) in prior null responders SVR12rates were higher in patients without NS5A polymorphisms at baseline In genotype 1a, DCV + SMV + RBV for 24 weeks achieved SVR12 of 66.7% in treatment-na ve patients. Null responders required intensification with PEG-IFN The DCV dose of 30 mg in combination with SMV provided lower than expected DCV exposure, however no relation with SVR12 DCV + SMV was well tolerated with or without RBV The standard 60mg dose of DCV will be utilized in all future studies involving DCV and SMV DCV + SMV + RBV is not recommended for the treatment of genotype 1a Other available options for HCV genotype 1b infection have surpassed this regimen in terms of efficacy LEAGUE-1 Zeuzem S, J Hepatol 2016; 64:292-300