SVR12 Rates in HCV Genotype 3 Cirrhotic Patients
Patients with HCV Genotype 3 and cirrhosis face challenges in achieving sustained virologic response. This study explores the efficacy of ABT-493 and ABT-530 with or without ribavirin in treatment-naïve individuals. The results show a promising 100% SVR12 rate, indicating a potential breakthrough in treating this difficult genotype in cirrhotic patients.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
100% SVR12 WITH ABT-493 AND ABT-530 WITH OR WITHOUT RIBAVIRIN IN TREATMENT-NA VE HCV GENOTYPE 3- INFECTED PATIENTS WITH CIRRHOSIS Paul Y Kwo1, David L Wyles2, Stanley Wang3, Fred Poordad4, Edward Gane5, Benedict Maliakkal6, Mitchell L Shiffman7, Teresa I Ng3, Chih-Wei Lin3, Ran Liu3, Jens Kort3, Federico J Mensa3 1Indiana University School of Medicine, Indianapolis, Indiana, USA; 2University of California San Diego, La Jolla, California, USA; 3AbbVie Inc., North Chicago, IL, USA; 4Texas Liver Institute, University of Texas Health Science Center, San Antonio, TX, USA; 5University of Auckland, Auckland, New Zealand; 6University of Rochester Medical Center, Rochester, New York, USA; 7Liver Institute of Virginia, Bon Secours Health System, Newport News and Richmond, VA, USA 51st Annual Meeting of the European Association for the Study of the Liver Barcelona, Spain 16 April 2016 1
Disclosures PY Kwo: Grant support/advisor: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck. DL Wyles: Research grants: AbbVie, Bristol-Myers Squibb, Gilead, Merck, Tacere (paid to the University of California Regents); Consultant: AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Merck. F Poordad: Grant/research support: AbbVie, Achillion Pharmaceuticals, Anadys Pharmaceuticals, Biolex Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Gilead Sciences, GlaxoSmithKline, GlobeImmune, Idenix Pharmaceuticals, Idera Pharmaceuticals, Intercept Pharmaceuticals, Janssen, Medarex, Medtronic, Merck, Novartis, Santaris Pharmaceuticals, Scynexis Pharmaceuticals, Vertex Pharmaceuticals, ZymoGenetics; Speaker: Gilead, Kadmon, Merck, Onyx/Bayer, Genentech, GSK, Salix, Vertex; Consultant/advisor: AbbVie, Achillion Pharmaceuticals, Anadys Pharmaceuticals, Biolex Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, GlobeImmune, Idenix, Merck, Novartis, Tibotec/Janssen, Theravance, Vertex. E Gane: Advisor: AbbVie, Gilead, Achillion Pharmaceuticals, Novartis, Roche, Merck, Janssen, Novira. Speaker: AbbVie, Gilead, Merck, Janssen, Novira, Alnylam. B Maliakkal: Speaker: AbbVie, Gilead, Bristol-Myers Squibb, Merck. ML Shiffman: Advisor: AbbVie, Bristol-Myers Squibb, Gilead, Merck; grant/research support: AbbVie, Beckman-Colter, Bristol- Myers Squibb, Conatus, Galactin, Gilead, Intercept, Intermune, Lumena, RocheDiagnostics; Speaker: AbbVie, Bayer, Gilead, Merck. S Wang, TI Ng, CW Lin, R Liu, J Kort, and FJ Mensa: employees of AbbVie and may hold stock or stock options. The design, study conduct, analysis, and financial support of the SURVEYOR-II (NCT02243293) study were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the content. All authors had access to all relevant data and participated in writing, review, and approval of this presentation. This presentation contains information on the investigational products ABT-493 and ABT-530. Medical writing support was provided by Douglas E. Dylla, PhD, of AbbVie. SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 2
HCV Genotype 3 (GT3) Higher rates of liver steatosis and an increased risk for hepatocellular carcinoma and fibrosis progression than other HCV genotypes1 Approximately 30% of HCV infections worldwide2 Now the most difficult-to-cure genotype, particularly in patients with cirrhosis Current EASL recommendations for GT3-infected patients with cirrhosis SOF + RBV for 24 weeks3 SOF + pegIFN/RBV for 12 weeks3 SOF + DCV + RBV for 24 weeks4,5 SVR12, sustained virologic response at post-treatment week 12; SOF, sofosbuvir; pegIFN, pegylated interferon; RBV, ribavirin; DCV, daclatasvir SVR12 79% 88% 85% 1. Andriulli A, et al. Aliment Pharmacol Ther. 2008; 28:397-404. 3. Foster GR, et al. Gastroenterology. 2015; 149:1462-70. 2. Messina JP, et al. Hepatology. 2015; 61:77-87. 4. Welzel TM, et al. AASLD 2015 5. Hezode C, et al. AASLD 2015 SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 3
Next Generation Direct-acting Antivirals ABT-530 Pangenotypic NS5A inhibitor ABT-493 Pangenotypic NS3/4A protease inhibitor High barrier to resistance Potent against common NS3 variants (eg., positions 80, 155, 168) and NS5A variants (eg., positions 28, 30, 31 and 93) Additive/synergistic antiviral activity Once-daily oral dosing Minimal metabolism and primary biliary excretion Negligible renal excretion (<1%) In vitro:1,2 Clinical PK & metabolism: ABT-493 identified by AbbVie and Enanta. 1. Ng TI, et al. Abstract 636. CROI, 2014. 2. Ng TI, et al. Abstract 639. CROI, 2014. SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 4
ABT-493 and ABT-530 Have Potent Activities Against All Major HCV Genotypes, Including GT3 Stable HCV GT3a Replicon EC50 nM 1.6 35 6.1 472 19 1162 NS3/4AProtease Inhibitor ABT-493 Grazoprevir1 GS-98572 Simeprevir3,4 Paritaprevir Asunaprevir5 NS5A Inhibitor ABT-530 Elbasvir7 Velpatasvir8 Ledipasvir9 Ombitasvir Daclatasvir10 Odalasvir11 MK-840812 pM 2 140 20 168,000 19 530 48 2 1. Lahser F, et al. AASLD, 2014. 5. McPhee F, et al. AAC, 2012. 9. Cheng G, et al. EASL, 2012 2. Taylor J, et al. EASL, 2015. 6. Yang H, et al. AAC, 2014. 10. Wang C, et al. AAC, 2014. 3. Olysio prescribing information. 7. Liu R, et al. AAC doi:10.1128/AAC.01390-15 11. ACHN R&D/Analyst Day 4. Chase R, et al. IAPAC, 2013. 8. Cheng G, et al. EASL, 2013. 12. Asante-Appiah E, AASLD, 2014. SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 5
ABT-530 Retains Antiviral Activity Against Common GT3 Single- Position NS5A Variants Fold Change in EC50 for GT3 NS5A Variants M28T A30K 0.4 1.1 NA >1000 NA 10 100 46 56 - 62 NA 50 423 NA NA NA NA NA NS5A Inhibitor Y93H 2.5 >1000 >100 2738 2752 486 6728 NA NA ABT-530 Ledipasvir1 Velpatasvir2 Daclatasvir3 Elbasvir4 Ombitasvir5 Odalasvir MK-8408 NA, not available 1. Hernandez D, et al. J Clin Virol, 2013. 4. Gane E, et al. EASL, 2015. 2. Doehle BP, et al. EASL, 2015. 5. Krishnan P, et al. AAC, 2015. 3. Wang C, et al. AAC, 2013:57:611-3. SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 6
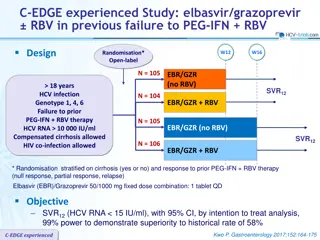
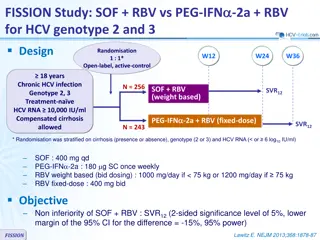
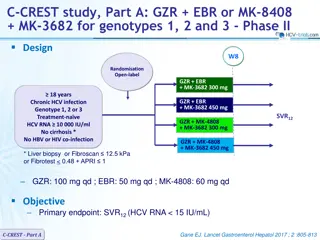
Results in Treatment-nave GT3-infected Patients Without Cirrhosis, and Objective 100 100 ABT-493 300 mg + ABT-530 120 mg resulted in mITT SVR12 rates of: 100 90 80 SVR12, % Patients 70 60 100% (28/28) with 8 weeks1 50 40 100% (26/26) with 12 weeks2 30 20 28 28 26 26 10 No virologic failures 0 ABT-493 ABT-530 300 120 300 120 8 Weeks 12 Weeks Objective: Evaluate the efficacy and safety of ABT-493 + ABT-530 in treatment-na ve GT3-infected patients with compensated cirrhosis and whether RBV improves response rates 1. Muir AJ, et al. PS098, EASL, 2016 2. Kwo PY, et al. AASLD, 2015. SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 7
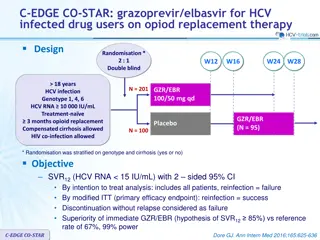
SURVEYOR-II Part 2 Study Design Partially randomised, open-label, multicentre phase 2 trial evaluating the dose combination of ABT-493 300 mg and ABT-530 120 mg identified in the dose-ranging part 1 of this study *RBV dosed once-daily. Blue diamond = randomised arms. SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 8
SURVEYOR-II Part 2 Study Design Partially randomised, open-label, multicentre phase 2 trial evaluating the dose combination of ABT-493 300 mg and ABT-530 120 mg identified in the dose-ranging part 1 of this study *RBV dosed once-daily. Blue diamond = randomised arms. SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 9
Key Eligibility Criteria and Endpoints Key inclusion criteria 18 to 70 years of age, inclusive HCV GT3 infection, HCV RNA >10,000 IU/mL Presence of compensated cirrhosis (Child-Pugh score 6) Liver biopsy (eg., METAVIR >3 or Ishak >4), or FibroScan 14.6 kPa, or FibroTest 0.75 with APRI >2 Key exclusion criteria Any prior HCV treatment Any prior history of hepatic decompensation HIV co-infection, albumin <LLN, platelet count <90 109/L Herbal supplements and potent P-gp inducers were prohibited Endpoints Efficacy: SVR12 (primary) and virologic failure Safety: adverse events (AEs) and laboratory abnormalities SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 10
Demographics and Patient Characteristics ABT-493 + ABT-530 ABT-493 + ABT-530 + RBV (N = 24) 18 (75) 22 (92) 57 (30 65) 27 (21 35) 24 (100) 6.3 (4.2 7.3) 116 (28 218) 157 (109 291) 4.2 (3.2 4.7) 3 (13) (N = 24) 13 (54) 23 (96) 55 (36 68) 27 (19 37) 22 (92) 6.4 (5.3 7.2) 100 (22 198) 140 (86 245) 4.1 (3.1 4.4) 5 (21) Male, n (%) White race, n (%) Age, median years (range) BMI, median kg/m2 (range) HCV GT3a*, n (%) HCV RNA, median log10 IU/mL (range) ALT, median U/L (range) Platelets, median 109/L (range) Albumin, median g/dL (range) Child-Pugh score = 6, n (%) * Genotype and subtype were determined by the Versant HCV Genotype Inno-LiPA Assay Version 2.0 or higher SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 11
Baseline Variants in NS3 and NS5A ABT-493 + ABT-530 ABT-493 + ABT-530 + RBV (N = 24) 8 (33) 2 2 4 (N = 24) 10 (42) 2 6 2 Any NS3 or NS5A variants, n (%) Both NS3 and NS5A variants, n NS3 only, n NS5A only, n Sequencing pending for 1 patient. Variants detected by population sequencing (detection threshold sensitivity of 15%). NS3 variants: A166S/T (n = 9) Y56F (n = 1) Q80K (n = 1) Q168K (n = 1) NS5A variants: Y93H (n = 4) A30K/T (n = 5) P58S (n = 2) L31M (n = 1) Variants detected by population sequencing (detection threshold sensitivity of 15%) at the following amino acid positions that confer resistance to at least 1 DAA in the inhibitor class were included in the analysis; they may not confer resistance to ABT-493 or ABT-530. NS3: 36, 56, 80, 155, 156, 166, and 168 NS5A: 24, 28, 29, 30, 31, 32, 58, 92, and 93 SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 12
100% SVR12 by ITT Analysis 100 100 100 90 80 SVR12, % Patients 70 60 50 40 30 20 24 24 24 24 10 0 ABT-493 + ABT-530 ABT-493 + ABT-530 + RBV SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 13
Summary of Adverse Events ABT-493 + ABT-530 ABT-493 + ABT-530 + RBV (N = 24) 20 (83) 2 (8) 0 (N = 24) 21 (88) 1 (4) 0 Event, n (%) Any AE Serious AE AE leading to study D/C Common AEs* Headache Fatigue Nausea URTI Dizziness Diarrhoea * Occurring in 10% of patients overall. D/C, discontinuation; URTI, upper respiratory tract infection. Tibia fracture on post-treatment day 15 assessed as unrelated to study drug. Anaemia on day 30 assessed as possibly related to RBV. Delusional disorder on post-treatment day 3 assessed as possibly related to ABT-493, ABT-530, and RBV following admitted amphetamine and alcohol use the same day. 3 (13) 2 (8) 2 (8) 4 (17) 2 (8) 5 (21) 8 (33) 6 (25) 6 (25) 2 (8) 4 (17) 0 SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 14
Laboratory Abnormalities ABT-493 + ABT-530 ABT-493 + ABT-530 + RBV (N = 24) 0 0 (N = 24) 0 0 Event, n ALT, grade 2 (>3 ULN)* AST, grade 2 (>3 ULN)* Total bilirubin Grade 2 (>1.5 3 ULN) Grade 3 (>3 10 ULN) Alk phos, grade 2 (>2.5 ULN) Haemoglobin Grade 2 (<10 8 g/dL) Grade 3 (<8 g/dL) * Post nadir 1 1 0 7 0 0 0 0 1 0 No ALT elevations 1 patient with elevated baseline bilirubin had a grade 3 total bilirubin elevation on day 44 that resolved post treatment SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 15
Summary and Conclusions 100% SVR12 with 12 weeks once-daily ABT-493 and ABT-530 in treatment-na ve GT3-infected patients with compensated cirrhosis regardless of RBV coadministration High efficacy was achieved regardless of the presence of baseline NS3 and/or NS5A variants This combination was well tolerated, with mostly mild AEs, few significant laboratory abnormalities, and no discontinuations due to AEs SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 16
Additional Data at EASL 12 weeks in GT1 with cirrhosis: 96% SVR12*; Poster SAT-135 8 weeks in GT1 or 2 without cirrhosis: 100% SVR12*; Poster SAT-157 8 weeks in GT3 without cirrhosis: 100% SVR12*; Oral PS098 12 weeks in GT4 6 without cirrhosis: 100% SVR12; Poster SAT-137 Trials in progress poster #THU-482: active-comparator study to daclatasvir + sofosbuvir in GT3-infected patients *mITT analysis SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 17
Based on These Data, ABT-493 and ABT-530 are Being Evaluated as a Pangenotypic RBV-free Regimen SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 18
Acknowledgements The authors would like to express their gratitude to the patients and their families who participated in this study. Additionally, we would like to acknowledge AbbVie s Clinical Virology team, all members of the SURVEYOR-II study team, and AbbVie s HCV Next Generation team who contributed to this study. SURVEYOR-II: ABT-493 + ABT-530 for Patients With Genotype 3 Infection and Cirrhosis | EASL | 16 April 2016 19