SOF + RBV Treatment for HCV Genotypes 2 and 3
The study investigates the efficacy of SOF + RBV treatment for HCV genotypes 2 and 3 in patients with chronic HCV infection. It includes baseline characteristics, patient disposition, SVR12 rates, multivariate analysis of predictors of SVR12, and factors associated with treatment response. The study's design, dosing regimens, outcomes, and results are detailed, providing valuable insights for managing HCV infections.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
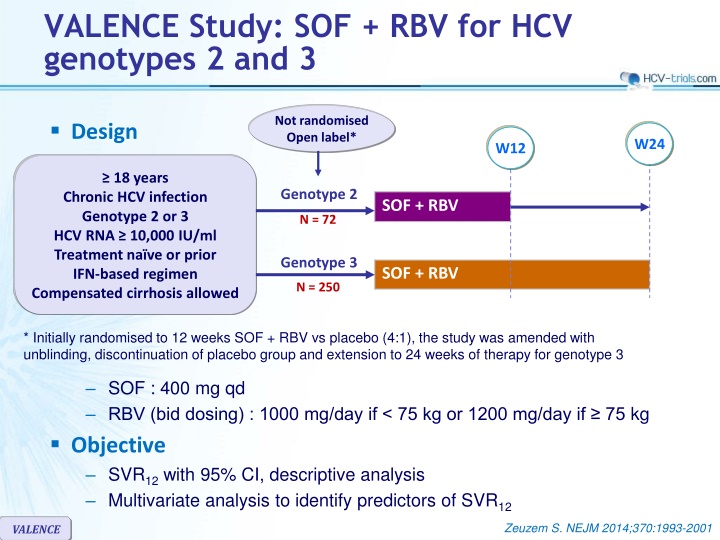
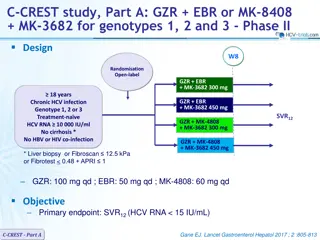
VALENCE Study: SOF + RBV for HCV genotypes 2 and 3 Not randomised Open label* Design W24 W12 18 years Genotype 2 Chronic HCV infection Genotype 2 or 3 HCV RNA 10,000 IU/ml Treatment na ve or prior IFN-based regimen Compensated cirrhosis allowed SOF + RBV N = 72 Genotype 3 SOF + RBV N = 250 * Initially randomised to 12 weeks SOF + RBV vs placebo (4:1), the study was amended with unblinding, discontinuation of placebo group and extension to 24 weeks of therapy for genotype 3 SOF : 400 mg qd RBV (bid dosing) : 1000 mg/day if < 75 kg or 1200 mg/day if 75 kg Objective SVR12with 95% CI, descriptive analysis Multivariate analysis to identify predictors of SVR12 Zeuzem S. NEJM 2014;370:1993-2001 VALENCE
VALENCE Study: SOF + RBV for HCV genotypes 2 and 3 Baseline characteristics and patient disposition Genotype 2 SOF + RBV 12W N = 73 Genotype 3 SOF + RBV 24W N = 250 Mean age, years 58 48 Female 45% 38% Race : white/black 89% / 7% 94% / 0 Body mass index, mean 26 25 IL28B CC genotype 33 % 34% HCV RNA log10IU/ml, mean (SD) Cirrhosis 6.5 0.7 6.3 0.7 15% 24% Previous IFN treatment 56% 58% Discontinued for side effects 4% 4% No response 14% 16% Relapse or breakthrough 38% 38% Discontinued treatment, N 0 4 (1 AE) In addition, 85 patients received placebo for a mean of 7 weeks before discontinuation of this group 11 patients, with genotype 3, initially randomised, received only 12 weeks of treatment Zeuzem S. NEJM 2014;370:1993-2001 VALENCE
VALENCE Study: SOF + RBV for HCV genotypes 2 and 3 SVR12(HCV RNA < 25 IU/ml) Genotype 2 - 12 weeks Genotype 3 - 24 weeks % 100 97 97 95 100 94 93* 94 92 90 87 85** 78 79 75 62 50 25 N 73 47 250 105 13 98 32 41 32 9 145 92 30 2 0 All TN TE NC C NC C All TN TE NC C NC C TN TE **95% CI: 80-89 TN TE * 95% CI: 85-98 TN : Treatment-na ve ; TE : Treatment-experienced ; NC : non-cirrhotic ; C : cirrhotic Zeuzem S. NEJM 2014;370:1993-2001 VALENCE
VALENCE Study: SOF + RBV for HCV genotypes 2 and 3 Multivariate analysis of factors associated with SVR12 in patients with genotype 3 OR (95% CI) p Baseline HCV RNA < 6 log10IU/ml Female 4.23 (1.21 - 14.81) 0.02 3.18 (1.22 - 8.31) 0.02 Absence of cirrhosis 3.46 (1.60 - 7.48) 0.002 < 50 years 2.82 (1.21 - 6.57) 0.02 Virologic breakthrough 1 in genotype 3 group (non adherent patient) Relapse in treatment completers 5 (7%) in genotype 2 group 5/11 (45%) in genotype 3 12W-group and 35 (14%) in genotype 3 24W-group Resistance testing (sequencing) Done in genotype 3 group with failures: No SOF-associated mutation (S282T) NS5B substitutions : V321A in 2 patients, L159F in 6 patients (no change in SOF susceptibility) Zeuzem S. NEJM 2014;370:1993-2001 VALENCE
VALENCE Study: SOF + RBV for HCV genotypes 2 and 3 Adverse events, N (%) Genotype 2 + 3 SOF + RBV 12W N = 84 1 0 Genotype 3 SOF + RBV 24W N = 250 1 10 (4%) AE leading to treatment discontinuation Serious adverse event AE occurring in > 10% in either group Headache Fatigue Pruritus Asthenia Nausea Insomnia Nasopharyngitis Dry skin Dyspnea Cough Diarrhea 29% 23% 24% 25% 31% 11% 5% 10% 14% 10% 5% 30% 30% 27% 21% 13% 16% 14% 12% 11% 11% 12% Zeuzem S. NEJM 2014;370:1993-2001 VALENCE
VALENCE Study: SOF + RBV for HCV genotypes 2 and 3 Summary The oral SOF + RBV regimen resulted in high rates of SVR12both in genotype 2 and genotype 3 infection High rates of response and low rates of relapse in all genotype 2 sub- groups with 12 weeks of SOF + RBV For genotype 3, SOF + RBV 24 weeks was associated with higher SVR12 than reported with the same regimen for 12 weeks or 16 weeks Identification of 4 possible predictors of SVR12among patients with genotype 3 infection: female sex, absence of cirrhosis, younger age, and a low viral load at baseline Severity and frequency of adverse events did not increase with longer duration of SOF + RBV treatment SOF + RBV has a high barrier to resistance Limitations Descriptive study Few number of patients with genotype 2 Zeuzem S. NEJM 2014;370:1993-2001 VALENCE