Stoichiometry: Quantitative Study of Reactants and Products
Stoichiometry is a fundamental aspect of chemical reactions, focusing on the quantitative relationships between reactants and products. It involves calculations using molar ratios, molar masses, and conversions between grams and moles. By understanding stoichiometry, one can determine the amounts of reactants required or products formed in a chemical reaction. Mole ratios play a crucial role in these calculations, helping to establish the relationship between different compounds involved. Practice problems and examples further demonstrate the application of stoichiometry in real-world scenarios, aiding in a better comprehension of this chemical concept.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
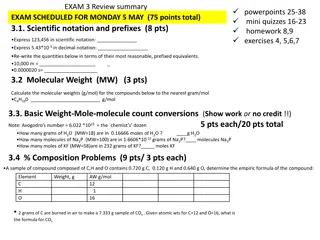
What is stoichiometry? Stoichiometry is the quantitative study of reactants and products in a chemical reaction.
What You Should Expect Given : Amount of reactants Question: how much of products can be formed. Example 2 A + 2B 3C Given 20.0 grams of A and sufficient B, how many grams of C can be produced?
What do you need? You will need to use molar ratios, molar masses, iii. balancing and interpreting equations, and conversions between grams and moles. i. ii. iv. Note: This type of problem is often called "mass-mass."
Steps Involved in Solving Mass-Mass Stoichiometry Problems Balance the chemical equation correctly Using the molar mass of the given substance, convert the mass given to moles. Construct a molar proportion (two molar ratios set equal to each other) Using the molar mass of the unknown substance, convert the moles just calculated to mass.
Mole Ratios A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound.
Example Reaction between magnesium and oxygen to form magnesium oxide. ( fireworks) 2 Mg(s) + O2(g) Mole Ratios: 2 : 1 2 MgO(s) : 2
Practice Problems 1) N2+ 3 H2---> 2 NH3 Write the mole ratios for N2to H2and NH3to H2. 2) A can of butane lighter fluid contains 1.20 moles of butane (C4H10). Calculate the number of moles of carbon dioxide given off when this butane is burned.
Mole-Mole Problems Using the practice question 2) above: Equation of reaction 2C4H10 + 13O2 8CO2 + 10H2O Mole ratio C4H10 1 : 1.2 : X By cross-multiplication, X = 4.8 mols of CO2 given off CO2 4 [ bases] [ problem]
Mole-Mass Problems Problem 1: 1.50 mol of KClO3decomposes. How many grams of O2will be produced? [k = 39, Cl = 35.5, O = 16] 2 KClO3 2 KCl + 3 O2
Three stepsGet Your Three steps Get Your Correct Answer Correct Answer Use mole ratio Get the answer in moles and then Convert to Mass. [Simple Arithmetic] Hello! If you are given a mass in the problem, you will need to convert this to moles first. Ok?
Lets go! 2 KClO3 2 1.50 X = 2.25mol Convert to mass 2.25 mol x 32.0 g/mol = 72.0 grams 2 KCl + 3 O2 : : 3 X Cool!
Try This: We want to produce 2.75 mol of KCl. How many grams of KClO3would be required? Soln KClO3 : 2 : 2 X : 2.75 X = 2.75mol In mass: 2.75mol X 122.55 g/mol = 337 grams zooo zimple! KCl
Mass-MassProblems There are four steps involved in solving these problems: Make sure you are working with a properly balanced equation. Convert grams of the substance given in the problem to moles. Construct two ratios - one from the problem and one from the equation and set them equal. Solve for "x," which is usually found in the ratio from the problem. Convert moles of the substance just solved for into grams.
Mass Mass- -Volume VolumeProblems Problems Just follow mass- mass problem to the penultimate level
Like this: Like this: There are four steps involved in solving these problems: Make sure you are working with a properly balanced equation. Convert grams of the substance given in the problem to moles. Construct two ratios - one from the problem and one from the equation and set them equal. Solve for "x," which is usually found in the ratio from the problem. Convert moles of the substance just solved for into Volume.
Conversion of mole to volume No of moles = Volume Molar volume Can you remember a similar equation?
Molar volume volume The molar volume is the volume occupied by one mole of ideal gas at STP. Its value is: 22.4dm3
Practice Problems Calculate the volume of carbon dioxide formed at STP in dm3' by the complete thermal decomposition of 3.125 g of pure calcium carbonate (Relative atomic mass of Ca=40, C=12, O=16) Solution: Convert the mass to mole: Molar mass of CaCO3 = 40 + 12 + (16 x 3) = 100gmol-1 Mole = mass/molar mass 3.125/100 = 0.03125mol
Practice Problems As per the equation, Mole ratio problem 1 0.03125mol : 1 X X = 0.03125mol of CO2 Convert mole to volume [slide 17] Volume = (0.03125x 22.4)dm3 = 0.7dm3
This powerpoint was kindly donated to www.worldofteaching.com http://www.worldofteaching.com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.