Study on the Effectiveness of SOF/VEL/VOX vs. SOF/VEL in HCV Treatment
Explore the POLARIS-4 study comparing the efficacy of SOF/VEL/VOX and SOF/VEL treatments in genotypes 1-6 of chronic HCV infection. Results show high SVR rates, especially in patients without cirrhosis, supporting the use of these regimens in DAA-experienced individuals. Baseline characteristics, SVR rates by genotype and cirrhosis status, and data on baseline RASs are provided.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
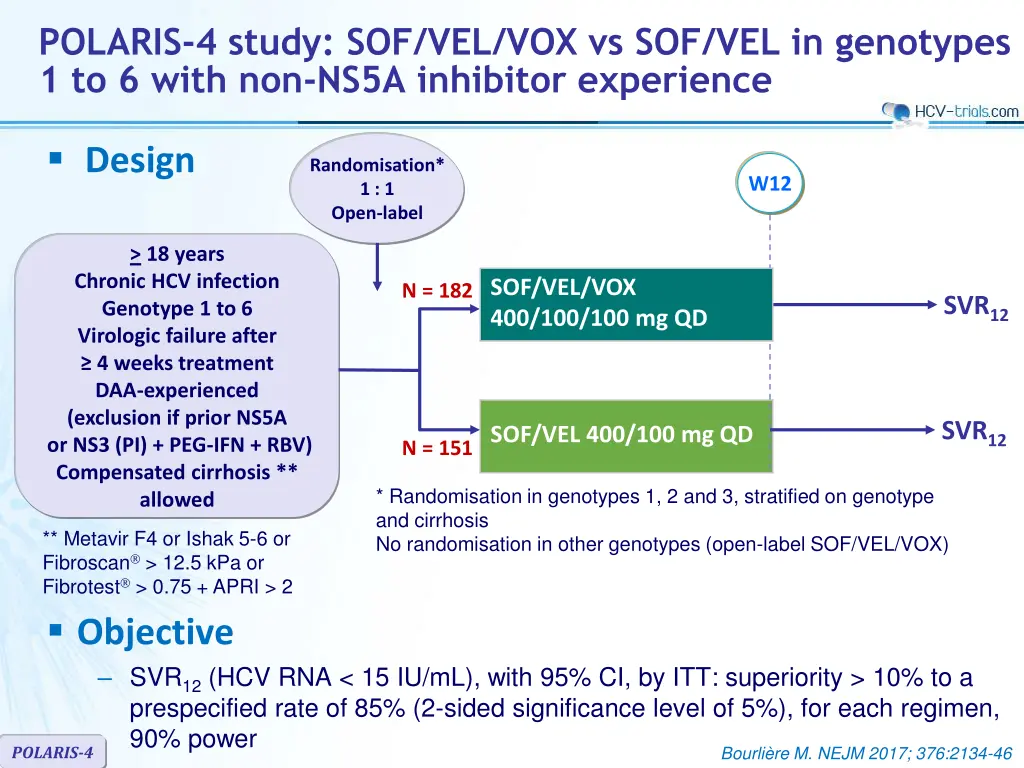
POLARIS-4 study: SOF/VEL/VOX vs SOF/VEL in genotypes 1 to 6 with non-NS5A inhibitor experience Design Randomisation* 1 : 1 Open-label W12 > 18 years Chronic HCV infection Genotype 1 to 6 Virologic failure after 4 weeks treatment DAA-experienced (exclusion if prior NS5A or NS3 (PI) + PEG-IFN + RBV) Compensated cirrhosis ** allowed SOF/VEL/VOX 400/100/100 mg QD N = 182 SVR12 SVR12 N = 151SOF/VEL 400/100 mg QD * Randomisation in genotypes 1, 2 and 3, stratified on genotype and cirrhosis No randomisation in other genotypes (open-label SOF/VEL/VOX) ** Metavir F4 or Ishak 5-6 or Fibroscan > 12.5 kPa or Fibrotest > 0.75 + APRI > 2 Objective SVR12(HCV RNA < 15 IU/mL), with 95% CI, by ITT: superiority > 10% to a prespecified rate of 85% (2-sided significance level of 5%), for each regimen, 90% power POLARIS-4 Bourli re M. NEJM 2017; 376:2134-46
POLARIS-4 study: SOF/VEL/VOX vs SOF/VEL in genotypes 1 to 6 with non-NS5A inhibitor experience Baseline characteristics SOF/VEL/VOX 12 weeks N = 182 57 21 88 6.3 46 SOF/VEL 12 weeks N = 151 57 25 87 6.3 46 Age, years, mean Female, % White, % HCV RNA, log10IU/mL, mean Cirrhosis, % Genotype, % 1a 1b 2 3 4 Previous DAA treatment , % NS5B inhibitor + NS3 inhibitor NS5B inhibitor NS3 inhibitor 2 regimens Most recent HCV treatment response, % No response / Relapse 30 13 17 30 10 29 14 22 34 0 25 74 1 39 25 72 2 40 4 / 94 8 / 87 POLARIS-4 Bourli re M. NEJM 2017; 376:2134-46
POLARIS-4 study: SOF/VEL/VOX vs SOF/VEL in genotypes 1 to 6 with non-NS5A inhibitor experience SVR12overall and by cirrhosis status, % (95% CI) SOF/VEL/VOX 12 weeks SOF/VEL 12 weeks 97.8 * (95-99) % 98 90.1 (84-94) 100 94 98 86 80 1 relapse 1 death 2 lost to follow-up 60 1 breakthrough 14 relapses 40 20 182 151 98 No cirrhosis 82 84 Cirrhosis 69 N= 0 Overall * p < 0.001 for superiority compared with prespecified 85% performance goal POLARIS-4 Bourli re M. NEJM 2017; 376:2134-46
POLARIS-4 study: SOF/VEL/VOX vs SOF/VEL in genotypes 1 to 6 with non-NS5A inhibitor experience SVR12by genotype, % SOF/VEL/VOX 12 weeks SOF/VEL 12 weeks % 100 100 98 97 96 100 95 96 89 85 80 60 40 20 54 44 24 22 31 33 54 52 19 0 N= 0 Genotype 1a Genotype 1b Genotype 2 Genotype 3 Genotype 4 POLARIS-4 Bourli re M. NEJM 2017; 376:2134-46
POLARIS-4 study: SOF/VEL/VOX vs SOF/VEL in genotypes 1 to 6 with non-NS5A inhibitor experience SVR12according to baseline RASs (15% cutoff) SOF/VEL/VOX 12 weeks * SOF/VEL 12 weeks ** N = 179 85/86 (98.8%) N = 151 67/75 (89.3%) No NS3 or NS5A RASs NS3 RAS only 39/39 (100%) 29/32 (90.6%) NS5A RAS only 40/40 (100%) 32/34 (94.1%) NS3 + NS5A RASs 4/4 (100%) 2/4 (50%) * All 22 patients with baseline NS5B RAS achieved SVR12 ; No treatment-emergent RASs in the patient who relapsed ** All 8 patients with baseline NS5B RAS achieved SVR12 ; 11/14 patients with virologic relapse developed Y93H or Y93C POLARIS-4 Bourli re M. NEJM 2017; 376:2134-46
POLARIS-4 study: SOF/VEL/VOX vs SOF/VEL in genotypes 1 to 6 with non-NS5A inhibitor experience Adverse events SOF/VEL/VOX 12 weeks N = 182 SOF/VEL 12 weeks N = 151 At least one adverse event, % Serious treatment-related adverse events, N Discontinuation due to adverse event, N (%) Death Adverse events in > 10% of patients, % Headache Fatigue Diarrhea Nausea 77 4 0 74 4 1 (< 1%) * 0 1 (< 1%) ** 27 24 20 12 28 28 5 8 Laboratory abnormalities, % Grade 3 Grade 4 5 6 < 1 < 1 *1 patient discontinued due to worsening headaches on study D49 ** 1 patient died of an opiate overdose on post-treatment D2 POLARIS-4 Bourli re M. NEJM 2017; 376:2134-46
POLARIS-4 study: SOF/VEL/VOX vs SOF/VEL in genotypes 1 to 6 with non-NS5A inhibitor experience Summary In a wide variety of DAA-experienced patients, excluding those pre-treated with NS5A inhibitor, with genotypes 1, 2, 3 or 4, SVR12was 98% for 12 weeks of SOF/VEL/VOX, meeting superiority criteria to prespecified 85% rate SVR12was 90% for 12 weeks of SOF/VEL Lower SVR12rate in cirrhotic patients (86% vs 96%) Baseline RASs did not impact outcome for SOF/VEL/VOX : SVR12 rates of 100% No treatment-emergent RASs in the patient who relapsed with SOF/VEL/VOX 79% (11/14) patients with virologic failure to SOF/VEL had emergence of Y93H or Y93C SOF/VEL/VOX and SOF/VEL were well tolerated SOF/VEL/VOX for 12 weeks provides a simple, safe, and effective single tablet, once daily treatment for NS5B-experienced patients POLARIS-4 Bourli re M. NEJM 2017; 376:2134-46