Understanding Ionic Compound Nomenclature and Writing Formulas
Learn about the nomenclature of ionic compounds, how to write their formulas using charge balance principles, and the different types of ionic compounds such as binary and polyatomic ions. Practice predicting formulas and names for various compounds.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
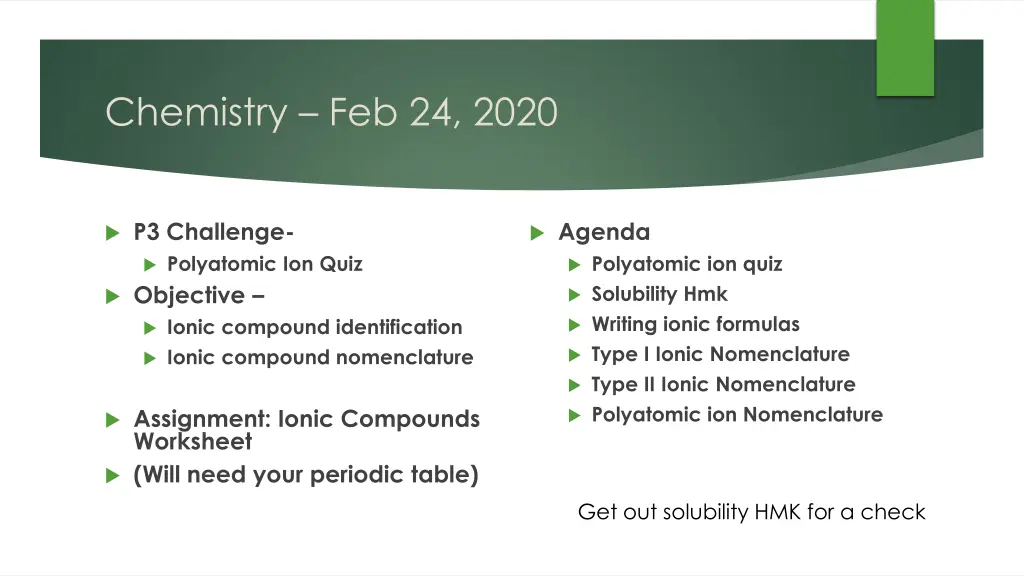
Chemistry Feb 24, 2020 P3 Challenge- Polyatomic Ion Quiz Objective Ionic compound identification Ionic compound nomenclature Agenda Polyatomic ion quiz Solubility Hmk Writing ionic formulas Type I Ionic Nomenclature Type II Ionic Nomenclature Polyatomic ion Nomenclature Assignment: Ionic Compounds Worksheet (Will need your periodic table) Get out solubility HMK for a check
Writing Ionic Formulas SHORT CUT From Lewis structures, we were able to predict ionic formulas. Because compounds are electrically neutral, one can determine the formula of a compound this simpler way: The charge on the cation becomes the subscript on the anion. The charge on the anion becomes the subscript on the cation. If these subscripts are not in the lowest whole-number ratio, divide them by the greatest common factor. Switch, Drop and Reduce
Writing Ionic Formulas Practice Predict ionic compounds that form using the exponent switch drop and reduce process to create a neutral formula. Formula always written with cation (metal) first Ex: compound formed by barium and chlorine Ex: compound formed by aluminum and oxygen
Writing Ionic Formulas Practice (cont) Ex: compound formed by sulfur and sodium Ex: compound formed by Fe+2 and oxygen Ex: compound formed by barium and hydroxide Use parentheses anytime you need more than one of a polyatomic ion
Chemical nomenclature overview Goal is to be able to write the formula of a named compound and to name a compound when given its formula. There are 7 nomenclature rules needed to learn this skill. 3 ionic rules (today) 1 covalent rule (next time) 3 acid rules (next time)
Three types of ionic compounds Type 1 - Binary compound (contains only two elements) and cation charge is known because atom can form only one type of ion. Metal forms only one type of ion: Groups 1A, 2A, Al3+, Ga3+, Zn2+, Cd2+, and Ag+ Type 2 Binary compound with variable charge cations. Transition metals and representative metals. Type 3 Polyatomic ion compound contains three or more elements contained within one or two polyatomic ions.
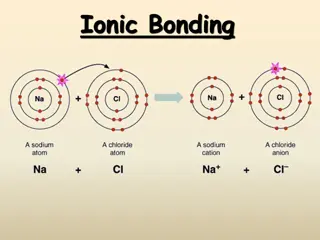
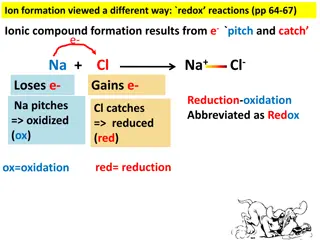
Type 1 Ionic Compounds Formed by electron transfer from a metal to a nonmetal Metal forms only one type of ion: Groups 1A, 2A, Al3+, Ga3+, Zn2+, Cd2+, and Ag+ Name as metal nonmetalide Name sodium chloride calcium oxide lithium fluoride beryllium nitride Formula Formula KBr Al2O3 ZnI2 Ag2S Name
Type 2 Ionic compounds Transition Metals Most transition metals and group 13-16 metals form multiple ions. Cr, Fe and Co form 2+ and 3+ ions Cu forms 1+ and 2+ ions Sn, Pb form 2+ and 4+ ions Named as metal (roman numeral for charge on metal) nonmetalide Name lead (II) oxide tin (IV) fluride copper (I) bromide iron (III) selenide Formula Formula CuO Au2S CoP Cr3N2 Name
Type 3 Ionic compounds polyatomic ions Formula will have more than two elements Often includes oxygen Often includes () around a polyatomic ion Name as cation anion Name sodium carbonate tin (II) cyanide ammonium nitrate barium sulfate Formula Formula CuOH Mg(ClO2)2 FePO4 LiN02 Name
Exit Slip - Homework Exit Slip: Name Ca(OH)2 Write the formula for Iron (II) sulfate What s Due? (Pending assignments to complete.) Ionic Compounds Worksheet What s Next? (How to prepare for the next day) Read Holt p159-175