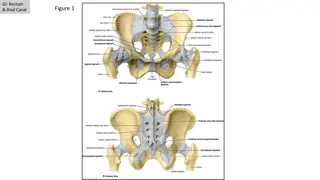
Anal Dysplasia and Cancer Screening Guidelines for Adults with HIV
Explore the latest clinical guidelines for screening anal dysplasia and cancer in adults with HIV. Learn about the importance of HPV vaccination, reducing morbidity, and early identification of precancerous lesions. Recommendations focus on prevention strategies and promoting overall health for individuals with HIV.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Screening for Anal Dysplasia and Cancer in Adults With HIV www.hivguidelines.org FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program
Purpose of This Guideline Purpose of This Guideline Increase the numbers of New York State residents with HIV who are screened and effectively treated for HPV-related anal and perianal dysplasia. Support the NYSDOH Prevention Agenda 2019-2024 by educating care providers on the importance of HPV vaccination and increasing the rate of 3-dose HPV immunization among individuals with HIV. Reduce the morbidity and mortality associated with HPV-related anal and perianal disease in individuals with HIV through early identification and treatment of potentially precancerous and cancerous lesions, when treatment is most likely to be effective. FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Key Points: Key Points: HPV HPV- -Related Anal Disease in Individuals With HIV Related Anal Disease in Individuals With HIV Lower rates of anal cancer screening for people of color have been described and represent inequities in health care. Missed opportunities for screening and prevention have been documented in 44% of individuals with anal cancer. Infection with more than 1 HPV type occurs more frequently among individuals with HIV, and such individuals can be at risk for cervical, vulvar, and perianal or anal SILs. The absence of HPV-related cervical disease in the genital tract does not eliminate the need to screen for anal dysplasia in cisgender women aged 45 years with HIV. For individuals diagnosed with vulvar cancer, initiate anal cancer screening with review of symptoms, DARE, and anal Pap testing. FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Recommendations: Recommendations: HPV Prevention HPV Prevention Given the increased lifetime risk of persistent HPV infection and increased prevalence of HPV-related cancers, clinicians should recommend the 3-dose nonavalent HPV vaccine series (0, 1 2, and 6 months) to all individuals with HIV aged 9 to 45 years regardless of CD4 cell count, prior cervical or anal screening results, HPV test results, HPV-related cytologic changes, or other history of HPV- related lesions. (A3) Clinicians should promote smoking cessation, optimal virologic control with antiretroviral therapy, and condom use for all patients with HIV, especially those at increased risk for anal cancer. (A3) FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Key Points: Key Points: HPV Prevention HPV Prevention It is important that clinicians inform patients with HIV about the risk of acquiring HPV and other STIs from close physical contact with the external genitalia, anus, cervix, vagina, urethra, mouth and oral cavity, or any other location where HPV lesions are present. Consistent and correct condom use remains an effective way to reduce the risk of transmission of most STIs, including HPV. However, it is important that clinicians inform patients that barrier protection, such as condoms and dental dams, may not fully protect against HPV. FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Recommendations: Recommendations: Screening for Anal Disease Screening for Anal Disease For all patients aged 35 years with HIV, regardless of HPV vaccination status, clinicians should: Inquire annually about anal symptoms, such as itching, bleeding, palpable masses or nodules, pain, tenesmus, or a feeling of rectal fullness. (A2) Perform a visual inspection of the perianal region. (A3) Provide information about anal cancer screening and engage the patient in shared decision-making regarding screening, including anal cytology before DARE. (A3) Perform DARE annually and whenever anal symptoms are present. (A*) Clinicians should evaluate any patient aged <35 years with HIV who presents with signs or symptoms that suggest anal dysplasia. (A3) For adults aged 35 years who have HIV and are cisgender men who have ever had sex with men (A3), are transgender women (A3), or are transgender men who have sex with men (B3), clinicians should perform or recommend annual (A3) anal cancer screening to identify dysplasia and precancerous and malignant lesions. FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Recommendations: Recommendations: Screening for Anal Disease, Screening for Anal Disease, continued continued For individuals who have been diagnosed with vulvar cancer or vulvar intraepithelial neoplasia grade 3, clinicians should initiate annual anal cancer screening within 1 year of diagnosis to identify dysplasia and precancerous and malignant lesions. (B1) For adults aged 45 years who have HIV and are cisgender women or are cisgender men or transgender men who have never had sex with men, clinicians should perform or recommend annual anal cancer screening to identify dysplasia and precancerous and malignant lesions. (A3) For individuals with HIV who have undergone solid organ transplant and have no other indication for earlier screening, clinicians should initiate anal cancer screening 10 years after transplant (as recommended for solid organ transplant recipients without HIV; see full guideline) if that occurs earlier than the recommended age for screening. (A3) Clinicians should engage in shared decision-making regarding discontinuing screening for anal cancer in individuals whose life expectancy is less than 10 years. (B3) For individuals who have 2 consecutive anal screenings that are negative for both high- risk HPV and high-grade dysplasia on cytology, clinicians should perform anal cancer screening every 3 years. (B3) FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Key Points: Key Points: Screening for Anal Disease Screening for Anal Disease All anal cancer screening strategies are acceptable, based on resources and available testing. Inform patients about the objective of anal cancer screening and risk prevention. It is important to discuss the specifics of the screening procedure and identify patient preferences to support informed decision- making about screening. The absence of high-risk HPV in the anal canal is associated with a low risk of high-grade dysplasia and anal cancer. For adults aged 45 years who have HIV and are cisgender women or are cisgender men or transgender men who have never had sex with men, a tailored and more acceptable approach to screening might be to offer hrHPV 16 testing alone as it has the highest negative predictive value; those who screen negative may repeat hrHPV screening every 2 to 3 years, whereas those who screen positive for HPV 16 would be referred to HRA. AUGUST 2022 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Anal Cancer Screening Strategies Anal Cancer Screening Strategies Screening Strategy Sensitivity Specificity Benefits and Limitations Anal cytology alone 88% (95% CI, 85 90) 30% (95% CI, 27 33) Has a high sensitivity but relatively low specificity and generates a large number of HRA referrals Anal cytology with hrHPV triage 85% (95% CI, 82 88) 47% (95% CI, 44 50) Generates fewer unnecessary HRAs than some other strategies but includes the second step of hrHPV determination hrHPV alone 96% (95% CI, 95 97) 27% (95% CI, 25 30) Has the highest sensitivity but lowest specificity and triggers the most HRA referrals hrHPV with anal cytology triage 85% (95% CI, 82 88) 48% (95% CI, 44 51) Generates fewer unnecessary HRAs than some other strategies but includes the second step of cytology Anal cytology with hrHPV cotesting 89% (95% CI, 86 91) 40% (95% CI, 37 44) An efficient strategy but requires coordination with laboratory services FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Performing an Anal Cytology Test Performing an Anal Cytology Test Perform an anal cytology test before using swabs for other STI testing, using lubricant, or performing DARE. A moistened nylon or polyester swab may be used to obtain an anal cytology sample according to the laboratory authority s collection instructions (cotton swabs should not be used). Instruct patients to refrain from performing an anal enema or douche, engaging in anal sex, or inserting any objects into the anus for 24 hours before cytologic screening. FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Recommendations: Follow Recommendations: Follow- -Up of Abnormal Anal Cancer Screening Results Anal Cancer Screening Results Up of Abnormal Clinicians should refer patients with abnormal anal cancer screening results to a care provider with experience performing HRA and follow up as indicated in Follow-Up of Anal Cancer Screening Results, by Screening Strategy. (A3) Clinicians should refer patients with suspected anal cancer determined by digital anorectal examination or histology to an experienced specialist for evaluation and management. (A3) Clinicians should perform cervical cancer screening for any individual who is not up to date with current cervical screening guidelines. (A3) FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Follow Follow- -Up of Anal Cancer Screening Results, Up of Anal Cancer Screening Results, Anal Cytology Alone Anal Cytology Alone FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Follow Follow- -Up of Anal Cancer Screening Results, Up of Anal Cancer Screening Results, Anal Cytology With hrHPV Triage Anal Cytology With hrHPV Triage FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Follow Follow- -Up of Anal Cancer Screening Results, Up of Anal Cancer Screening Results, hrHPV Testing Alone hrHPV Testing Alone FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Follow Follow- -Up of Anal Cancer Screening Results, Up of Anal Cancer Screening Results, hrHPV Testing With Anal Cytology Triage hrHPV Testing With Anal Cytology Triage FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Follow Follow- -Up of Anal Cancer Screening Results, Up of Anal Cancer Screening Results, Anal Cytology With hrHPV Cotesting Anal Cytology With hrHPV Cotesting FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Recommendations: Treatment and Follow Recommendations: Treatment and Follow- -Up Clinicians should perform or refer for post-treatment follow-up with repeat HRA at 6 months in patients who have been successfully treated for anal HSILs. (A3) Clinicians should base follow-up after a patient's first post-treatment HRA and biopsy on the most recent histopathology findings (see Follow-Up of Anal Cancer Screening Results, by Screening Strategy). (A3) For patients with a history of HSILs, clinicians should continue annual HRA or annual anal cancer screening, with referral for HRA if screening results are abnormal, as long as life expectancy exceeds 10 years (A3), until 2 consecutive anal screenings are negative for both high-risk HPV and high-grade dysplasia on cytology, after which clinical assessment and anal cancer screening should be performed every 3 years. (B3) Clinicians should immediately refer patients diagnosed with anal cancer to an oncologist or surgeon trained in the management of anal cancer. (A2) Clinicians should closely monitor patients with anal cancer in collaboration with the oncologist after definitive treatment for cancer. (A3) Up FEBRUARY 2025 NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Need Help? Need Help? NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org
Access the Guideline Access the Guideline www.hivguidelines.org > Screening for Anal Dysplasia and Cancer in Adults With HIV Also available: Printable pocket guide and PDF NYSDOH AIDS Institute Clinical Guidelines Program www.hivguidelines.org