ASTRAL-3 Study: SOF/VEL vs. SOF+RBV in Genotype 3 Randomisation
This study compares SOF/VEL versus SOF+RBV treatment in patients with genotype 3 chronic HCV infection. The objective is to achieve SVR12 with non-inferiority of SOF/VEL with a lower bound of 95% CI for difference of -10% and 94% power. Baseline characteristics, patient disposition, SVR12 rates, and outcomes by cirrhosis or treatment history are discussed.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
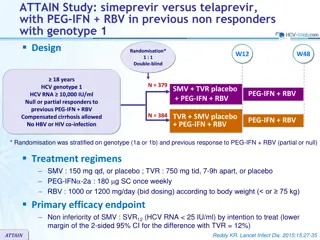
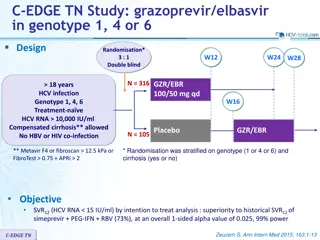
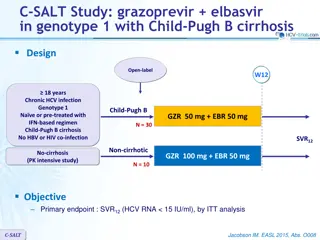
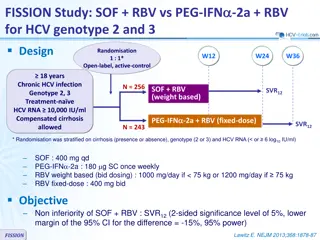
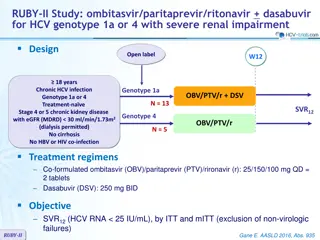
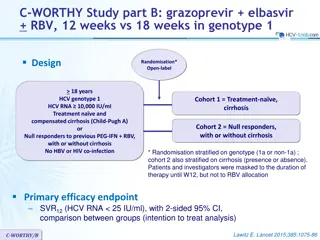
ASTRAL-3 study: SOF/VEL vs SOF + RBV in genotype 3 Design Randomisation* 1 : 1 Open-label W12 W24 SOF/VEL 400/100 mg qd > 18 years N = 250 Chronic HCV infection Genotype 3 Na ve or pre-treatment with IFN-based regimen Compensated cirrhosis allowed** No HBV or HIV co-infection SVR12 SOF + RBV N = 250 * Randomisation was stratified on prior treatment (na ve or experienced) and cirrhosis (yes or no) ** Metavir F4 or Ishak 5-6 or Fibroscan > 12.5 kPa or Fibrotest > 0.75 and APRI > 2 RBV (in 2 divided doses): 1000 mg if < 75 kg or 1200 mg/day if 75 kg Objective SVR12 (HCV RNA < 15 UI/ml),by ITT : non-inferiority of SOF/VEL with a lower bound of 95% CI for difference of - 10%, 94% power ; if non-inferiority, test for superiority with significance level of 0.05 ASTRAL-3 Foster GR. N Engl J Med 2015; 373: 2608-17
ASTRAL-3 study: SOF/VEL vs SOF + RBV in genotype 3 Baseline characteristics and patient disposition SOF/VEL 12 weeks N = 277 49 39% 90% 6.2 0.72 38% 29% 26% SOF + RBV 24 weeks N = 275 50 37% 87% 6.3 0.71 40% 30% 26% Age, years, mean Female White HCV RNA, log10IU/ml, mean IL28B CC Cirrhosis Treatment experienced Response to previous HCV treatment No response Relapse Discontinuation, N Lack of efficacy Adverse event Lost to follow-up Non adherence Withdrew consent Death 28% 72% 2 1 0 0 1 0 0 34% 66% 21 1 9 4 2 3 2 ASTRAL-3 Foster GR. N Engl J Med 2015; 373: 2608-17
ASTRAL-3 study: SOF/VEL vs SOF + RBV in genotype 3 SVR12, % (95% CI) * 95.3 % (92.1-97.5) 100 80.4 (75.2-84.9) 80 60 40 20 277 275 0 SOF/VEL 12 weeks SOF + RBV 24 weeks *adjusted absolute difference : 14.8 (95% CI : 9.6 to 20.0) ; p < 0.001 = superiority SVR12according to baseline NS5A RAVs in SOF/VEL group Absent, N = 231 : SVR12= 97.4% Present, N = 43, SVR12= 88.4% (84% if Y93H) ASTRAL-3 Foster GR. N Engl J Med 2015; 373: 2608-17
ASTRAL-3 study: SOF/VEL vs SOF + RBV in genotype 3 SVR12by cirrhosis or treatment history SOF/VEL SOF + RBV 97 (94-99) 97 100 91 90 94-99) 87 86 (83-96) (81-96) (82-92) (81-91) 80 66 63 (55-76) (51-75) 60 22 relapses 6 other 7 relapses 7 relapses 40 4 relapses 2 other 16 relapses 8 other 1 non-response 23 relapses 2 other 15 relapses 13 other 4 relapses 2 other 20 197 187 80 83 206 204 71 71 0 No cirrhosis Cirrhosis Treatment-na ve Treatment-experienced SVR12in cirrhosis : SOF/VEL group : 93% if treatment-na ve ; 89% if treatment-experienced SOF + RBV group : 73% if treatment-na ve ; 58% if treatment-experienced ASTRAL-3 Foster GR. N Engl J Med 2015; 373: 2608-17
ASTRAL-3 study: SOF/VEL vs SOF + RBV in genotype 3 Characteristics of patients receiving SOF/VEL who relapsed Resistance-associated variants HCV RNA (log10 IU/ml) Timing of virologic failure HCV NS5B Age, sex, race GT Cirrhosis IL28B treatment history NS5A Baseline and follow-up W12 Baseline FU W12 BL 56, F, White 3a Yes CC 6.9 FU W4 Na ve Y93H (15.2%) Y93H (> 99%) None 58,M, White 3a Yes CC 6.3 FU W12 Experienced None Y93H (> 99%) None 61, M, White 3a Yes CT 6.0 FU W12 Na ve Y93H (> 99%) Y93H (> 99%) None 61 / M /White 3a No TT 5.5 FU W4 Experienced None Y93H (> 99%) None 50, M, White 3a No CT 6.5 FU W4 Na ve Y93H (> 99%) Y93H (> 99%) None 56, M, White 3a Yes TT 6.1 FU W4 Experienced None Y93H (> 99%) None 45, M, White 3 No CC 6.9 FU W4 Experienced Y93H (2.8%) Y93H (> 99%) None A30K (> 99%) Y93H (> 99%) 46, M, White 3a Yes CT 6.1 FU W4 Experienced A30K (> 99%) None 57, M, White 3a Yes CT 6.3 FU W4 Na ve None Y93H (> 99%) None 56, M, White 3a Yes CT 6.3 FU W4 Experienced None Y93H (> 99%) None 39, M, White 3a No CC 6.6 FU W12 Experienced None GT1a reinfection ASTRAL-3 Foster GR. N Engl J Med 2015; 373: 2608-17
ASTRAL-3 study: SOF/VEL vs SOF + RBV in genotype 3 Adverse events, N (%) SOF/VEL 12 weeks N = 277 88% 6 (2%) 12 (4%) 0 0 SOF + RBV 24 weeks N = 275 95% 15 (5%) 23 (8%) 9 (3%) 3 (< 1%) At least one adverse event Serious adverse events Grade 3-4 adverse events Discontinuation due to adverse event Death Adverse events in > 10% of patients Headache Fatigue Insomnia Nausea Nasopharyngitis Irritability Cough Pruritus Dyspepsia Grade 3-4 laboratory abnormalities Hemoglobin < 10 g/dl Lymphocyte count < 500/mm3 Platelet count 25,000-50,000/mm3 Total bilirubin > 2.5 mg/dl 32% 26% 11% 17% 12% 8% 5% 3% 3% 7% 0 3 1 0 32% 38% 27% 21% 12% 15% 13% 13% 11% 17% 4% 4 1 3 ASTRAL-3 Foster GR. N Engl J Med 2015; 373: 2608-17
ASTRAL-3 study: SOF/VEL vs SOF + RBV in genotype 3 Summary Rates of SVR12in every subgroup of patients with HCV genotype 3 were substantially higher among those who had received 12 weeks of SOF/VEL compared to 24 weeks of SOF + RBV, including patients with cirrhosis and previous treatment failure Overall SVR12of 95% with SOF/VEL for 12 weeks versus 80% with SOF + RBV for 24 weeks (p < 0.001) 91% SVR12rate in patients with cirrhosis Limitation : small number of black patients However, the rate of SVR12was 88% among patients who had NS5A RAVs at baseline and 97% among those who did not, with the lowest rate (84%) observed among patients with the Y93H variant at baseline SOF/VEL was well tolerated and, compared with SOF + RBV, lacked toxicities commonly associated with RBV For patients with HCV genotype 3 infection, SOF/VEL for 12 weeks represents an improvement over standard treatment with 24 weeks of SOF + RBV, with a simple and highly effective regimen, together with shorter duration of treatment and fewer side effects, owing to the removal of RBV from the regimen ASTRAL-3 Foster GR. N Engl J Med 2015; 373: 2608-17