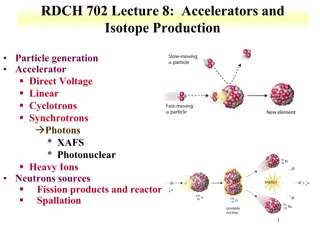
Production of Radiopharmaceuticals
Radiopharmaceuticals play a crucial role in medical imaging, with a significant portion containing Tc-99m that requires chemical modification. Various Tc-labeled radiopharmaceuticals like DTPA, HIDA, MIBI, and others are used for imaging. Desirable properties of radionuclides include half-life suitability, gamma ray emission, and easy attachment to pharmaceuticals. Technetium (Tc) chemistry involves its electro-positivity, variable oxidation states, complex formation, and radioactive isotopes with distinct decays. Tc-99m is commonly obtained from a 99Mo/99mTc generator as Sodium pertechnetate.
Production of Radiopharmaceuticals
PowerPoint presentation about 'Production of Radiopharmaceuticals'. This presentation describes the topic on Radiopharmaceuticals play a crucial role in medical imaging, with a significant portion containing Tc-99m that requires chemical modification. Various Tc-labeled radiopharmaceuticals like DTPA, HIDA, MIBI, and others are used for imaging. Desirable properties of radionuclides include half-life suitability, gamma ray emission, and easy attachment to pharmaceuticals. Technetium (Tc) chemistry involves its electro-positivity, variable oxidation states, complex formation, and radioactive isotopes with distinct decays. Tc-99m is commonly obtained from a 99Mo/99mTc generator as Sodium pertechnetate.. Download this presentation absolutely free.
Presentation Transcript
Production of Radiopharmaceuticals Alkhair Adam Khalil, B. Pharm, M. Pharm College of Pharmacy Karary University Department of Pharmaceutical Chemistry
86% of Radiopharmaceuticals are made of Tc-99m & 2/3 of them require chemical modification of 99mTc in their formulation.
Some 99mTc-labled Radiopharmaceuticals DTPA(Diethylene Triamine Penta-Acetic Acid) HIDA (Hepatobiliary iminodiacetic acid) MIBI (Methoxyisobutylisonitrile)(Myocardial perfusion imaging) HMPAO (Hexamethylpropylene amine oxime) MAA (Macroaggregates Albumin) MDP (Methylene Diphosphonate) DMSA (Dimercaptosuccinate) HAS (Human Serum Albumin) TMA (Thiomalic acid)
Desirable properties of a radionuclide for imaging i. A physical half life (short effective) of a few hours, similar to the time From preparation to injection. If the half life is too short, much more activity must be prepared than is actually injected. ii. Decay to a stable daughter or at least one with a very long half life. iii. Emit gamma rays but no (alpha or beta particles) i.e decays by EC , IT.
iv. Emit gamma rays of energy 50 300 KeV and ideally about 150 keV, high enough to exit the patient but low enough to be easily collimated and easily measured . v. Ideally emit mono energetic gamma rays so that scatter can be eliminated by energy discrimination vi. Be easily and firmly attached to pharmaceutical at room temperature but not affect its metabolism. vii. Be readily available on the hospital site. viii.Have a high specific activity.
Technetium Chemistry (Tc) Atomic number = 43, Metal. Electron configuration: 1s2,2s2,2p6,3s2,3p6,4s2,3d10,4p6,5s2,4d5 Thus due to Unsaturated d orbit , it: Is electro-positives: (Oxidation No -1 to +7, stable ones +4 & +7) Has Variable combing power (d-orbital accept different no electrons) Forms complexes (by electron pair donation) All Tc isotopes are radioactives with different decays modes & half lives. Examples (Tc-102(5sec), Tc-97m(90days) ,Tc-98m (60 days),Tc-99m (6 hours) Obtained in solution (10-9 molar) from 99Mo/99mTc generator as Sodium pertechnetate NaTcO4
Technetium Chemistry (Tc) Sodium pertechnetate NaTcO4, is the most stable in water & air ( chemically inert) .It is an oxidizing agent (Ox. No = +7). Size & charge of (TcO4-) is comparable to perchlorate (ClO4-) & iodide (I-) with same biodistribution behavior in GIT & Renal system, (TcO4-) is trapped in the thyroid. Reactions : 1. Oxidation-Reduction Reactions. 2. Ligand exchange. 3. Chelation. 4. Protein binding. 5. Sulfur based reduction reactions.
Technetium Chemistry (Tc) 1. Reduction : ( 1st step to prepare Tc-99m labeled Radiopharmaceuticals) TcO4- is reduced to ( +5 or +3) using Stannous chloride (SnCl2) Then the radionuclide (Tc-99m) can be complexed with variety of compounds to control its biologic behavior. Reduction & complex formation depends on: 1-Redox potentials ( Tc state & SnCl2). 2- Conc. of reducing agent(crucial), complexing agent & Tc. 3- complex stability ability to hold onto the Tc. 4-Time after production & temp. 5- order of adding reagents( formulation & preparation)
Reducing agents: In descending order: Sn(II) the BEST, Fe(II), Ascorbate, S2O2 in acid solution , conc. Hcl (complicated by S colloids formation e.g. Na2S2O3 + 2HCl 2NaCl +H2O + SO2 + S ), organic thiols -SH , NaBH4, electrolysis. To reduce TcO4- (VII) to TcO2(IV) using Sn(II): Sn2+ Sn4+ +2e ------------------------X3 TcO4- + 8H+ +3e TcO2+ 4H2O ------X2 Overall reaction: 2TcO4- + 3Sn2+ + 16H+ 3Sn4+ + 2TcO2 + 8H2O
Technetium Chemistry (Tc) 2. Ligand exchange: Unfilled , d- orbitals , can be filled with electrons from Ligands such as: Anions: Cl- ,F-, OH-, DMSA, DTPA, HIDA. Cations: MIBI. Molecules: H2O, ROH, CO, HMPAO,ECD. Groups: NH2, -SH, -PO43- Peptides, Receptor Ligands ,Antibodies etc.
Technetium Chemistry (Tc) 3. Chelation: Chelating agents are complexing agents with more than one ligand or complexing site , (octopus) enfolding metal ion e.g. reduced Tc. They have their own biologic behavior ,which the central metal ion does not affect ( carrier). E.g. EDTA(6 sites ) , DTPA( 8 sites). Excreted through the kidneys
If TcO4- is added to excess Sn2+-DTPA mixture 50:50 mixture of Tc3+-DTPA + Tc4+-DTPA. If Sn2+ is added to a mixture of TcO4- & DTPA only Tc4+- DTPA complex. ( order of addition). Other chelates include: phosphate - PO43- ,pyrophosphate (two phosphates in a P-O-P linkage), phosphonate H3PO3 ( MDP) & Phytate (six-fold dihydrogenphosphate ester of inositol) So Tc can form different complexes forms with the same ligand or chelate depending on its oxidation state , therefore to maintain the required oxidation state we should control the ionic power(pH) of the reducing agent & the conditions of the reaction & use minimum conc. of 99mTc (??-?molar) .
Technetium Chemistry (Tc) 4. Protein Binding: Protein molecule has many ligands chelating agent. E.g. HSA-Tc , MAA-Tc & microspheres of A Tc & now Nano colloid of HAS-Tc . etc.
Technetium Chemistry (Tc) 5. Sulfur Based Reduction Reactions: Thiols , Thioamino acids, Penicillamine, under basic condition react with TcO4- to form complexes that excreted through the kidneys. E.g. DMSA
Tc-99m Radiopharmaceuticals are either: 1. Tc-99m - tagged compounds: The target molecule or particle is directly labeled with Tc-99m & its biologic distribution is then traced, they include, high molecular weight protein , cells , colloids. Examples: Tc-99m Sulphur colloids , used to study the functions of liver , spleen & bone marrow. Tc-99m- MAA (Albumin macroaggregates) used for studying lung perfusion Tc-99m-RBC used for studying blood pool. Tc-99m-tagged Antibodies used for studying soft tissues tumors.
2. Tc-99m essential class compounds: They have coordination bonds between Tc-99m & the ligand to give labeled product. Their biologic distribution depends on their physical properties . Their biologic distribution differs with the structure of the labeled compound that depends directly on: 1- Oxidation state of Tc-99m. 2- Functional groups of the ligand molecules. 3- Tc -99m-core configuration & the ligand. They are used for diagnosing the disease of the tissues themselves e.g. liver ,kidneys, heart, bones etc.
Desired Properties of Radiopharmaceutical I. Localize largely and quickly in the target the tissues of diagnostic interest( high target to non target activity ratio) II . Be eliminated from the body with an effective half life similar to the duration of the examination, to reduce the subsequent dose to the patient. III . Non-toxic. IV . Form a stable product both in vitro and in vivo. V . Be readily available and affordable per patient dose.
Examples Technetium radiopharmaceuticals: 99mTc-pertechnetate : use in Thyroid, salivary gland 99mTc-MAA Lung scanning 99mTc-DTPA and MAG3 for Renal scanning 99mTc-HMPAO for Cerebral blood flow scanning. Non-technetium radiopharmaceuticals: Thallium-201, Gallium-67 citrate, 111Indium (WBC, Octreotide). Radiopharmaceuticals for PET: 18F-Fluorodeoxyglucose, 15O water, 13N-ammonia
Mechanisms of localization of Radiopharmaceuticals Passive diffusion: 99mTc-DTPA in brain imaging, 99mTc DTPA aerosol and 133Xe in ventilation imaging, 111In-DTPA in cisternography. Ion exchange: uptake of 99mTc-phosphonate complexes in bone. Capillary blockage: 99mTc-macroaggregated albumin (MAA) particles trapped in the lung capillaries. Phagocytosis: removal of 99mTc-sulfur colloid particles by the reticuloendothelial cells in the liver, spleen, and bone marrow. Active transport: 131I uptake in the thyroid, 201Tl uptake in the myocardium.
Cell sequestration: sequestration of heat- damaged 99mTc-labeled RBCs by the spleen. Metabolism:18F-FDG uptake in myocardial and brain tissues. Receptor binding: 11C-dopamine binding to the dopamine receptors in the brain. Compartmental localization: 99mTc-labeled RBCs used in the gated blood pool study. Antigen-antibody complex formation: 131I-, 111In-, and 99mTc-labeled antibody to localize tumours. Chemotaxis:111In-labeled leukocytes to localize infections.
Examples of Kit formulation 99mTc Radiopharmaceutical SULPHUR COLLOID: Solution A: 0.34 g Na2S2O3.5H2O 0.37 g gelatin 0.562 g K2HPO4.3H2O 0.118 g EDTA Dissolve in 50 ml water with slight heat. Autoclave for 15 min.
Solution B: 0.5 N HCl Autoclave for 15 min. Solution C: 0.175 g Na2HPO4.2H2O 0.6 g NaOH Dissolve in 50 ml water .Do not sterilize in (Autoclave). Pass through Millipore 0.22 micron.
Labeling: Add 4-7 ml Pertechnetate (TcO4- ) to solution A. Add 1 ml solution B . Heat in boiling water for 15 min , cool ,add 1 ml of solution C. QC: ITLC-SG (Instant Thin Layer Chromatography) . Solvent saline 0.9% Rf TcO4- :1.0 Colloid :0.0
MDP Methylene DiPhosphonate Dissolve 0.75 g MDP in 70 ml water . Add 0.18 g Ascorbic Acid and 0.2 g of NaCl. Add 0.2 ml SnCl2 solution 0.9 g in 5 ml conc. HCl . Adjust pH to 5.8-6.0. Dilute to 100 ml . Sterilize through Millipore . Dispense 1 ml in 10 ml vials. Lyophilize at 4 oC. Stopper under vacuum. Labeling: Add 3-7 ml TcO4- and shake.
QC: ITLC-SG Rf acetone NaCl 0.9% Solvent Tc-MDP 0.0 1.0 TcO4-1.0 1.0 Reduced Tc 0.0 0.0
Some 99mTc-labled Radiopharmaceuticals DTPA(Diethylene Triamine Penta-Acetic Acid) HIDA (Hepatobiliary iminodiacetic acid) MIBI (Methoxyisobutylisonitrile)(Myocardial perfusion imaging) HMPAO (Hexamethylpropylene amine oxime) MAA (Macroaggregates Albumin) MDP (Methylene Diphosphonate) DMSA (Dimercaptosuccinate) HAS (Human Serum Albumin) TMA (Thiomalic acid)
99mTc-MDP Technetium (99mTc) medronic acid (99mTc-MDP) is a pharmaceutical product used in nuclear medicine to localize bone metastases
99mTc-DTPA(Diethylene Triamine Penta-Acetic Acid) Used for Renography
99mTc-sestaMIBI The drug is a coordination complex consisting of the radioisotope technetium-99m bound to six (sesta = 6) methoxyisobutylisonitrile (MIBI) ligands. Sestamibi is taken up by tissues with large numbers of mitochondria and negative plasma membrane potentials, It is mainly used to image the myocardium (heart muscle) and other applications.
99mTc-HMPAO (Hexamethylpropylene amine oxime) Other name Technetium (99mTc) exametazime. Used by nuclear medicine physicians for the detection of altered regional cerebral perfusion in stroke. Exametazime
99mTc-DMSA (Dimercaptosuccinate) Used to assess renal morphology, structure and function. Dimercaptosuccinic acid 99mTc-DMSA