Efficacy of Ombitasvir/Paritaprevir/Ritonavir for HCV Genotype 1 Treatment
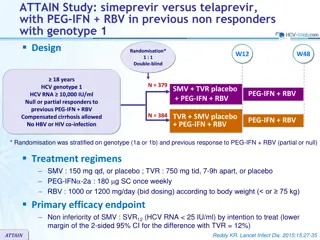
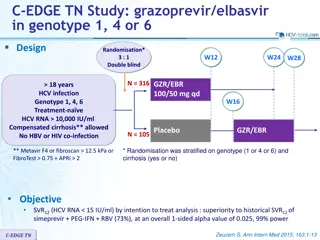
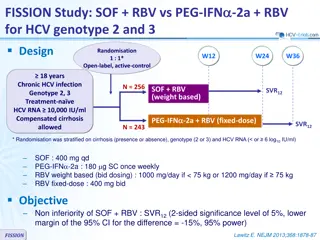
The PEARL-III and PEARL-IV studies assessed the efficacy of ombitasvir/paritaprevir/ritonavir in treating HCV genotype 1 infection. The trials focused on treatment-naïve individuals without cirrhosis and demonstrated non-inferiority of sustained virologic response compared to telaprevir-based regimens. The treatment regimens, endpoints, virologic failure criteria, and exploratory analyses were described in detail. Overall, the study emphasized the importance of this combination therapy for achieving optimal outcomes in HCV management.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
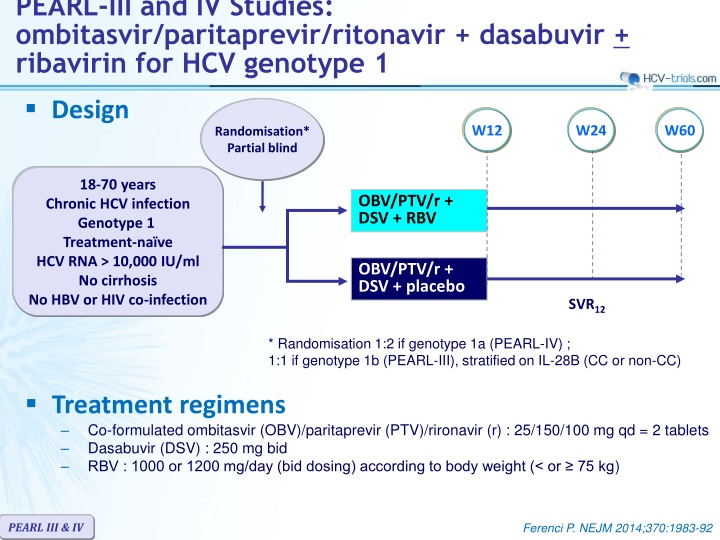
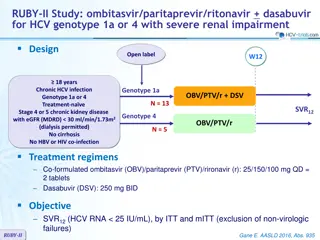
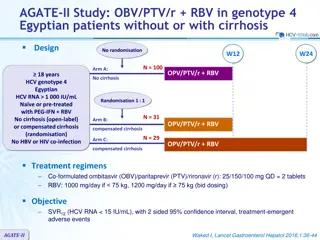
PEARL-III and IV Studies: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1 Design W12 W24 W60 Randomisation* Partial blind 18-70 years Chronic HCV infection Genotype 1 Treatment-na ve HCV RNA > 10,000 IU/ml No cirrhosis No HBV or HIV co-infection OBV/PTV/r + DSV + RBV OBV/PTV/r + DSV + placebo SVR12 * Randomisation 1:2 if genotype 1a (PEARL-IV) ; 1:1 if genotype 1b (PEARL-III), stratified on IL-28B (CC or non-CC) Treatment regimens Co-formulated ombitasvir (OBV)/paritaprevir (PTV)/rironavir (r) : 25/150/100 mg qd = 2 tablets Dasabuvir (DSV) : 250 mg bid RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or 75 kg) PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
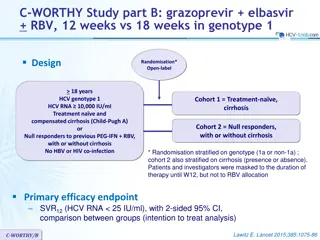
PEARL-III and IV Studies: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1 Efficacy endpoints Sustained virologic response (HCV RNA < 25 IU/mL) 12 weeks after end of treatment, with two-sided 95% CI, modified ITT analysis Non-inferiority of SVR assessed vs estimated rate of SVR24with a telaprevir- based regimen for genotype 1a : 72%, 95% CI: 68 to 75 for genotype 1b : 80%, 95% CI: 75 to 84 Non-inferiority (primary end-point) established if lower bound of the 95% CI for the SVR greater than the upper bound of the 95% CI for SVR of telaprevir-based therapy minus 10.5%, i.e. 65% in PEARL-IV and 73% in PEARL-III ; power 95% Superiority in PEARL-IV (genotype 1a) if lower margin of the 95% CI for the SVR12 > 75% ; in PEARL-III (genotype 1b) if lower margin of the 95% CI for the SVR12 > 84% ; power 90% Non inferiority of RBV vs placebo in both studies : SVR12rate, with lower margin of the 95% CI for the difference = -10.5% ; power 95% Trial conduct Hemoglobin and hematocrit results blinded to investigators, unless criteria for virologic failure or relevant predefined toxicity were met PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
PEARL-III and IV Studies: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1 HCV RNA : COBAS TaqMan real-time RT PCR assay, v 2.0 (Roche) Virologic failure : 2 consecutive HCV RNA > 1 log10IU/ml above the nadir at any time during treatment, HCV RNA 25 UI/ml at all assessments during treatment among patients who received at least 6 weeks of treatment, confirmed HCV RNA 25 IU/ml after a level < 25 IU/ml during treatment Virologic relapse : confirmed HCV RNA 25 IU/ml between the end of treatment and 12 weeks after the last dose of study drug among patients who completed treatment and had an HCV RNA < 25 IU/ml at the final visit during the treatment period Exploratory analysis : stepwise logistic-regression model to assess the association between the rate of SVR12and continuous and categorical subgroup variables PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
PEARL-IV Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1a Baseline characteristics and patient disposition 3D + RBV N = 100 3D + placebo N = 205 Mean age, years 51.6 51.4 Female 30% 37.1% Race : white/black 86.0% / 10.0% 83.4% / 12.7% Body mass index, mean (SD) 26.9 4.0 26.7 4.3 Metavir fibrosis score : F0-F1 / F2 / F3 63% / 21% / 16% 64% / 17% / 19% IL28B CC genotype 31.0% 30.7% HCV RNA log10IU/ml, mean (SD) 6.64 0.50 6.53 0.68 Discontinued treatment, N 0 11 For adverse event / for virologic failure - 2 / 6 PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
PEARL-III Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1b Baseline characteristics and patient disposition 3D + RBV N = 210 3D + placebo N = 209 Mean age, years 48.4 49.2 Female 49.5% 58.9% Race : white/black 94.3% / 4.8% 94.2% / 4.8% Body mass index, mean (SD) 25.8 3.8 26.1 4.2 Metavir fibrosis score : F0-F1 / F2 / F3 71% / 18% / 11% 68% / 23% / 10% IL28B CC genotype 21.0% 21.1% HCV RNA log10IU/ml, mean (SD) 6.29 0.77 6.33 0.67 Discontinued treatment, N 1 1 Withdrew consent 1 1 PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
PEARL-III and IV Studies: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1 SVR12(HCV RNA < 25 IU/ml) PEARL-IV (GT 1a) PEARL-III (GT 1b) Outcomes for patients without SVR12 3D + RBV 3 3D + placebo 20 3D + RBV 3D + placebo 3D + RBV 3D + placebo 99.5 % PEARL-IV, N On-treatment virologic failure Relapse Early discontinuation Missing data 99.0 97 100 90.2 1 6 1/98 0 1 3D + RBV 1 10/194 3 1 3D + placebo 2 75 SVR12 50 PEARL-III, N On-treatment virologic failure Relapse Early discontinuation Missing data 25 1 0 N 100 205 210 209 0 0 0 0 0 0 2 - 6.8% - 0.5% (95% CI: -12.0 to -1.5) superiority, and (95% CI: -2.1 to 1.1) non-inferiority thresholds (vs TVR) PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
PEARL-IV Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1a Multivariate analysis of factors associated with increased SVR IL28B CC genotype (p = 0.03) Resistance testing (population sequencing) 18 virologic failures (on-treatment or relapse) Resistance, N = 18/18 Most frequent variants : D168V (NS3), M28T and Q30R (NS5A), S556G (NS5B) PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
PEARL-IV Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1a Adverse events and laboratory abnormalities, n (%) 3D + RBV 92.0% 0 3 (3.0%) 3D + placebo 82.4% 2 1 (0.5% p Any adverse event AE leading to treatment discontinuation Serious AE AE occurring in > 10% in either group Headache Fatigue Pruritus Nausea Insomnia Diarrhea Laboratory abnormalities Hemoglobin < LLN Hemoglobin 10 g/dl Total biliubin > 3 x ULN ALT > 5 x ULN AST > 5 x ULN 0.03 25.0% 46.0% 10.0% 21.0% 17.0% 14.0% 28.3% 35.1% 5.9% 13.7% 7.8% 16.1% 0.02 42.0% 4.0% 3.0% 1.0% 0 3.9% 0 0.5% 0.5% 0 < 0.001 0.01 PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
PEARL-III Study: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1b Adverse events and laboratory abnormalities, n (%) 3D + RBV 80.0% 0 4 (1.9%) 3D + placebo 67.0% 0 4 (1.9%) p Any adverse event AE leading to treatment discontinuation Serious AE AE occurring in > 10% in either group Headache Fatigue Pruritus Nausea Insomnia Diarrhea Laboratory abnormalities Hemoglobin < LLN Hemoglobin 10 g/dl Total biliubin > 3 x ULN ALT > 5 x ULN AST > 5 x ULN 0.003 24.3% 21.4% 11.9% 11.0% 9.0% 4.3% 23.4% 23.0% 5.3% 4.3% 3.3% 6.2% 12.8% 3.4% 0 0.5% 0 0 0.02 0.02 0.02 51.2% 9.0% 5.7% 1.0% 0 < 0.001 < 0.001 0.003 PEARL III & IV Ferenci P. NEJM 2014;370:1983-92
PEARL-III and IV Studies: ombitasvir/paritaprevir/ritonavir + dasabuvir + ribavirin for HCV genotype 1 Summary After 12 weeks of treatment with ombitasvir/paritaprevir/ritonavir and dasabuvir with or without RBV, 90.2% to 99.5% of previously untreated patients with HCV genotype 1 infection and no cirrhosis had a SVR12 Response rates in all treatment groups were superior to the historical response rate with a PEG-IFN-containing telaprevir- based regimen In genotype 1b, SVR12 was > 99% with or without RBV A total of 18 patients with genotype 1a had virologic failure, and only 2/18 received RBV. The use of RBV in genotype 1a confers an additional benefit Regardless of whether the antiviral regimen included RBV, the rate of discontinuation of the study drugs owing to adverse events was low (<1%) PEARL III & IV Ferenci P. NEJM 2014;370:1983-92